Shilpa and twin sister Shweta Iyer have been working on splitting water to extract hydrogen for many years. In November, 2012 the Port Jefferson Station high school students won regional finalist honors in the Siemens Competition in Math, Science & Technology, and netted $1,000 each. They were awarded the Grand Prize in the Long Island Science and Engineering Fair in March, and were sent to Phoenix, Arizona in May to compete in the Intel International Science and Engineering Fair, where they won fourth place and $500 each. Their winnings go into college funds which they will invest in the fall.
What has won these young women international recognition? Perhaps it’s their work in creating a catalyst for the production of hydrogen – from literally dirt cheap material that advisor James Muckerman credits with being, “the best performing, non-noble-metal-containing hydrogen evolution catalyst yet known – and even better than bulk platinum metal.”
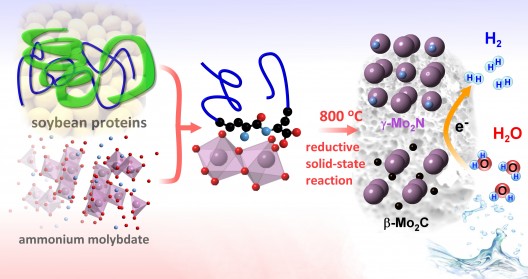
This illustration depicts the synthesis of a new hydrogen-production catalyst from soybean proteins and ammonium molybdate. Mixing and heating the ingredients leads to a solid-state reaction and the formation of nanostructured molybdenum carbide and molybdenum nitride crystals. The hybrid material effectively catalyzes the conversion of liquid water to hydrogen gas while remaining stable in an acidic environment. Illustration: Brookhaven National Laboratory
The young women entered the U. S. Department of Energy’s Brookhaven National Laboratory as part of an internship program under the guidance of chemist Wei-Fu Chen. Working with James Muckerman, Etsuko Fujita, and Kotaro Sasaki, they explored ways of using sunlight to develop alternative fuels.
Brookhaven’s newsletter explains, “Their ultimate goal is to find ways to use solar energy—either directly or via electricity generated by solar cells—to convert the end products of hydrocarbon combustion, water and carbon dioxide, back into a carbon-based fuel. Dubbed “artificial photosynthesis,” this process mimics how plants convert those same ingredients to energy in the form of sugars. One key step is splitting water, or water electrolysis.”

Wei-Fu Chen, Shweta Iyer, James Muckerman, Sasaki Kotaro, Etsuko Fujita, and Shilpa Iyer at Brookhaven National Laboratories
Sasaki expanded on the idea. “By splitting liquid water (H2O) into hydrogen and oxygen, the hydrogen can be regenerated as a gas (H2) and used directly as fuel. We sought to fabricate a commercially viable catalyst from earth-abundant materials for application in water electrolysis, and the outcome is indeed superb.”
Usually this type of catalysis requires high-priced materials such as platinum, at $1,345 per troy ounce. The Iyer twins found an amalgam of soy beans and molybdenum salt (MoSoy) made a better-performing option.
Soy beans cost about $12.00 per 60-pound bushel, or about 20 cents a pound – certainly an incentive to explore their energy-producing abilities outside of teriyaki. Molybdenum costs $10.52 a pound, less than a dollar per troy ounce, or about 1/1,500th the cost of platinum.
This excited their lab partners, who see commercial reality at the end of this rainbow. Etsuko Fujita, head of the artificial photosynthesis group in the Brookhaven Chemistry Department, sees MoSoy as “A very promising route to making a carbon-containing fuel is to hydrogenate carbon dioxide (or carbon monoxide) using solar-produced hydrogen,”
Peering through high-resolution transmission microscopes, Brookhaven scientists and the twins observed the anchored MoSoy nanocrystals on 2D graphene sheets. They found that, “Though not quite as active as commercially available platinum catalysts, the high performance of graphene-anchored MoSoy was extremely encouraging to the scientific team.”
Brookhaven elaborates on where this research might go. “’The direct growth of anchored MoSoy nanocrystals on graphene sheets may enhance the formation of strongly coupled hybrid materials with intimate, seamless electron transfer pathways, thus accelerating the electron transfer rate for the chemical desorption of hydrogen from the catalyst, further reducing the energy required for the reaction to take place,’ Sasaki said.
“The scientists are conducting additional studies to gain a deeper understanding of the nature of the interaction at the catalyst-graphene interface, and exploring ways to further improve its performance. They have produced a paper, published in the journal, Energy & Environmental Science (available online).
“In the paper, the authors—including the two high-school students—conclude: ‘This study unambiguously provides evidence that a cheap and earth-abundant transition metal such as molybdenum can be turned into an active catalyst by the controlled solid-state reaction with soybeans…The preparation of the MoSoy catalyst is simple and can be easily scaled up. Its long-term durability and ultra-low capital cost satisfy the prerequisites for its application in the construction of large-scale devices. These findings thus open up new prospects for combining inexpensive biomass and transition metals…to produce catalysts for electro-catalytic reactions.’”
Very few scientific papers include a term such as “unambiguously,” showing the certainty of the teams’ findings.
Additional collaborators in this research were Chiu-Hui Wang and Yimei Zhu of Brookhaven Lab.
