Hydrogen continues on its course of always being five to ten years away as a cheap, viable storage mechanism for energy. The ideal of driving a car that emits only water vapor (or flying an airplane that zooms about on a few pounds of H2) seems like an ever-distant dream.
Tina Casey, writing for Gas2.com reports on Rice University solution using stinky ammonia that might clear the air for hydrogen, though. She explains that the October 8th celebration of the fourth annual Hydrogen and Fuel Cell Day was great for natural gas stakeholders, since the gas is the primary source today for hydrogen. Her headline indicates this could become a leading way to store and extract H2: “Forget the Hydrogen Economy, Here Comes the Ammonia Economy.”
So Desirable. So Hard to Get.
Casey explains the big drawbacks to this market – fugitive greenhouse gas emissions and natural gas’s non-sustainable nature. Another factor, the often high cost of producing H2, adds to the difficulties facing its ready availability. She notes, “Renewable hydrogen is not a particularly new thing. The real challenge is getting the cost of renewable hydrogen down to parity with fossil-sourced H2.”
The Houston Chronicle reported on another Rice hydrogen researcher, James M. Tour, whose laboratory pursues different means of obtaining cheap hydrogen. “’How do you store electricity? You have to make it into a fuel – something you can put into a bottle and ship around,’ Tour said. ‘Hydrogen is a great way to store electricity.’”
The Chronicle adds another concern that Tour expressed: “The cheapest and most common way to produce hydrogen is to use heat, steam and a nickel catalyst to split methane into hydrogen and carbon dioxide. But generating one carbon dioxide molecule for every two hydrogen molecules makes little environmental sense if the goal is to cut greenhouse gas emissions.” Methane is indeed problematic, with its release during fracking for natural gas not uncommon. Clouds of escaping methane caused the Aliso Canyon evacuation in 2015, displacing 25,000 residents for over four months and costing over a billion dollars in damage settlements.
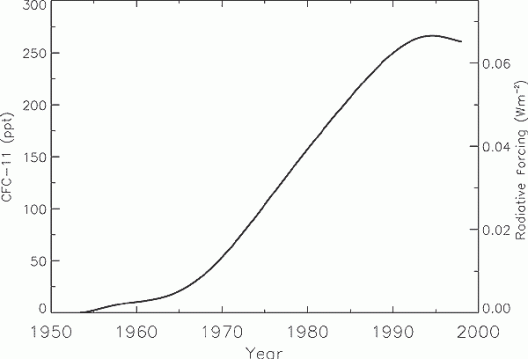
Global mean CFC-11 (CFCl3) tropospheric abundance (ppt) from 1950 to 1998 based on smoothed measurements and emission models. CFC-11’s radiative forcing is shown on the right axis. Source: IPCC
Global mean CFC-11 (CFCl3) tropospheric abundance (ppt) from 1950 to 1998 based on smoothed measurements and emission models. CFC-11’s radiative forcing is shown on the right axis.
Environmental issues aside, the U. S. Department of Energy looks for cost parity with gasoline, Casey adds. “In order for fuel cell electric vehicles to be competitive, the total untaxed, delivered and dispensed, cost of hydrogen needs to be less than $4/gge. A gge, or gasoline gallon equivalent, is the amount of fuel that has the same amount of energy as a gallon of gasoline. One kilogram of hydrogen is equivalent to one gallon of gasoline.”
The Ammonia Economy?
Casey dispenses with several ways of producing renewable H2, including pulling it from landfill and wastewater gas, biomass gasification, and splitting water with an electrical current, and turns to Rice University’s potential offering, hydrogen from ammonia.
The counter-intuitive approach seems more reasonable the more it is explained. Question: Why produce renewable hydrogen to make renewable ammonia, and then turn around and pull the hydrogen back out? Answer: You can transport renewable ammonia fairly easily and reduce the “delivered and dispensed” part of your fuel costs. She suggests that we “Think of ammonia as an energy storage system and you get to the “Neighborhood Energy Station” concept, in which a facility the size of a conventional gas station provides for local energy needs.
Your editor thought of the dangers of a tanker-size load of ammonia cracking open after a collision on the freeway, and thought that would be a good time to be elsewhere. Fortunately, the Ammonia Energy Association (there is such a thing), cites two reports from Rise National Laboratory and Quest Consultants, Inc. that, “Ammonia risk levels are acceptable and similar to current fuels.”
How to Make Cheap Hydrogen?
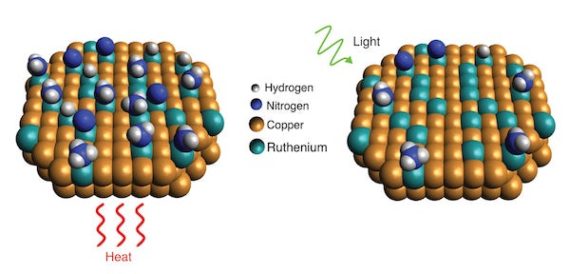
Getting that renewable hydrogen, according to one Rice University research team, uses a new catalyst, heat, and light to extract the desirable element. According to their paper in the journal Science, their catalysts rely on “the new catalytic nanoparticles, which are made mostly of copper with trace amounts of ruthenium* metal. Tests showed the catalyst benefited from a light-induced electronic process that significantly lowered the “activation barrier,” or minimum energy needed, for the ruthenium to break apart ammonia molecules.” Not only does this budget approach work for breaking up ammonia molecules, but it may find applications in “commercial ammonia-based systems that generate hydrogen fuel on demand.”
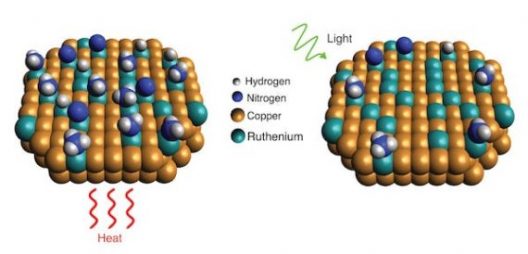
“Scientists with Rice’s Laboratory for Nanophotonics have shown how a light-driven plasmonic effect allows catalysts of copper and ruthenium to more efficiently break apart ammonia molecules, which each contain one nitrogen and three hydrogen atoms. When the catalyst is exposed to light (right), resonant plasmonic effects produce high-energy “hot carrier” electrons that become localized at ruthenium reaction sites and speed up desorption of nitrogen compared with reactions conducted in the dark with heat (left)” by LANP/Rice University.
Heating the catalyst and shining light on it makes all the activation barriers fall more easily, apparently. Still, the question remains – are we able to make enough hydrogen this way, and in turning it into ammonia for transport, able to extract it in such an efficient way that we get the benefits we seek?
More on Plasmonics
Namoi Hala, Stanley C. Moore Professor of Electrical and Computer Engineering at Rice and director of the school’s Laboratory for Nanophotonics, has an interesting blog on her laboratory’s many specialties, featuring “A Plethora of Plasmonics from the Laboratory for Nanophotonics at Rice University.” The blog includes explanations of the science of plasmonics, active plasmonics, Single Nanoparticle and Single Molecule Spectroscopy, Line Shape Engineering, and Theranostics** and Biomedical Applications. These areas of research are almost as obscure as quantum physics, and this definition of plasmonics probably yields meaning only after lengthy and deep reflection.
A kinder, gentler definition might help. “Plasmonics is the name given (in 2000) to a discipline for exploiting the resonant interaction obtained under certain conditions between electromagnetic radiation… and free electrons at the interface between a metal and a dielectric material (e.g. air or glass). This interaction generates electron density waves called plasmons or surface plasmons.”
For more details check out the article “Quantifying hot carrier and thermal contributions in plasmonic photocatalysis” in the journal Science.
*Ruthenium is a chemical element with symbol Ru and atomic number 44. A rare transition metal, it belongs to the platinum group of the periodic table.
**Theranostics is a new field of medicine which combines specific targeted therapy based on specific targeted diagnostic tests. With a key focus on patient centred care, theranostics provides a transition from conventional medicine to a contemporary personalized and precision medicine approach. The theranostics paradigm involves using nanoscience to unite diagnostic and therapeutic applications to form a single agent, allowing for diagnosis, drug delivery and treatment response monitoring.