Stanford researchers, working with scientists at Korea’s Ulsan National Institute of Science and Technology (UNIST) and China’s National Laboratory of Microstructures (Nanjing), School of Electronic Science and Engineering, at Nanjing University, have squeezed carbon as flat (if not flatter than) as graphene and poked lots of well-sized holes in it to make designer battery and supercapacitor components. Professor Zhenan Bao led the efforts at Stanford.
The combined teams’ paper, “Ultrahigh Surface Area Three-Dimensional Porous Graphitic Carbon from Conjugated Polymeric Molecular Framework,” appeared as a cover article in the May 18 edition of the journal ACS Central Science.
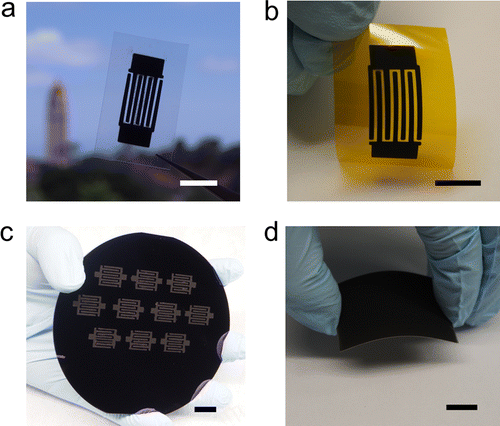
HPG carbon electrodes and supercapacitors fabricated on different substrates. (a) An interdigital supercapacitor made by spray coating HPG carbon ink on a gold-coated (50 nm) PET film. (b) A flexible supercapacitor with interdigital electrodes made by spray coating HPG carbon ink on an Al-coated (50 nm) Kapton polyimide film with 50 nm Al conducting layer. (c) Ten supercapacitors with interdigital electrodes fabricated at the same time on a silicon wafer using a removable PDMS (polydimethylsiloxane) mask. (d) A 4 cm × 5 cm size electrode (thickness of ∼100 μm) made by blade coating HPG carbon slurry on a Ti substrate. Scale bar, 1 cm (a–d). Photos and caption from ACS paper
The paper explains, “High surface area porous carbon materials are of great technological importance due to their diverse functionalities and excellent physical/chemical robustness. Their high electronic conductivity, large surface area, and good chemical and electrochemical stability are of particular interest for electrochemical energy storage devices, such as electrochemical capacitors (or supercapacitors) and batteries.”
High surface area gives more space for electrolyte to interact with anodes and cathodes, and porosity enables ions free transit between the two. Graphene, one atom thick and covering 2,675 square meters (28,793.5 square feet) per gram, exhibits excellent conduction, for example.
Co-lead author Zheng Chen expands on that idea. “For supercapacitors, the ideal carbon material has a high surface area for storing electrical charges, high conductivity for transporting electrons and a suitable pore architecture that allows for the rapid movement of ions from the electrolyte solution to the carbon surface.”
Paradoxically, the material has a greater surface area per gram than graphene, which according to the Graphene Council has a theoretical limit 2,630 square meters per gram (already exceeded). The Council says, “That’s the theoretical limit and no one has achieved anything higher than 1520m2/g for a supercapacitor electrode. Meanwhile there already exist activated carbon-based electrodes that have surface areas of 3000m2/g.” The paradox comes from the fact that graphene is already one atom thick. Since it can’t have extra depth, where does the extra area come from? Your editor awaits schooling in atomic-level physics.
Even more impressive, the team achieved a world record surface area per gram – 4,073 (43,841 square feet or about 1.5 football fields) with their carbon material.
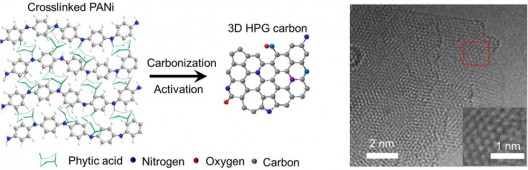
The reputedly simple process for converting hydrogels to electrodes. olyaniline (PANI) is a conducting polymer of the semi-flexible rod polymer family, and its carbonization and specialization through chemical processing helps make the desired designer carbon. Note the resulting materials (right) resemble graphene
Performance comes from “the capability of carbon materials to interact with ions and to transport electrons.” Since materials used in batteries are “generally synthesized from coal or biomass” such as coconut shells or rice husks, processing usually includes expensive purification tasks. Soft or hard templates used to create pores sized to allow ion flow and electron transfer add to the complexity and expense of manufacture. To avoid these extra-cost adders in manufacture, the researchers turned to available hydro polymer gels normally used to make contact lenses.
The gels are apparently easier than coconut shells to carbonize and chemically activate, and depending on process temperatures, can be spread as thick or thin as desired and size pores to different sizes, which allows flow of different materials. Chemical processing helps further size and align those pores to customize their behavior. Other carbons provide a “tortuous” route for ions to travel, and these designer carbons straighten the path.
The carbons designed by the three-university group have high surface area, small carbon particle size, open pore structure and good conductivity, all desirable in batteries and supercapacitors. Their summary explains, “We have developed a scalable synthetic approach to prepare 3D porous graphitic carbon from conjugated polymer molecular framework by a one-step synthesis from low-cost starting materials.” They report electrical conductivity three times that of conventional carbon supercapacity electrodes, and better stability in multiple cycles.
Similar results for lithium-sulfur batteries show promise for designer carbon as a low-cost way to tune energy storage devices for exacting requirements.
