Chemists at Boston College in Chestnut Hill, Massachusetts, “Have achieved a series of breakthroughs in their efforts to develop an economical means of harnessing artificial photosynthesis by narrowing the voltage gap between the two crucial processes of oxidation and reduction, according to their paper, “Hematite-Based Water Splitting with Low Turn-on Voltage,” published this week in the journal Angewandte Chemie.”
With your editor wishing he’d paid more attention in high-school chemistry, a quick search came up with simple definitions of oxidation and reduction.
- Oxidation is gain of oxygen.
- Reduction is loss of oxygen.
When reduction and oxidation take place simultaneously, this is known as a redox reaction.
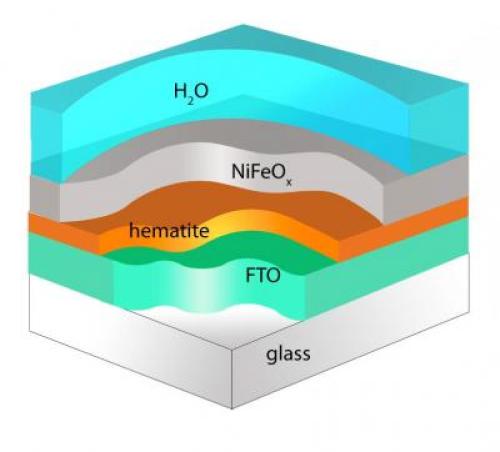
Researchers at Boston College report that modifying the surface of hematite with a nickel iron oxide coating produces an increase in cathode photovoltage of nearly four-tenths of a volt. That’s nearly enough energy to put an economical method of artificial photosynthesis within reach. Issustraton: Angewandte Chemie
These types of reactions take place in leaves producing plant energy from sunlight, and researchers are closing in on duplicating the reactions at a level which will make artificial photosynthesis an inexpensive, practical way to collect and store energy.
So far, they’ve managed to produce 80 percent of the necessary voltage levels from their unique photoanodes and photocathodes. “Many researchers have been trying to harvest solar energy and directly store it in chemical bonds. Solar panels can harvest energy, but economical storage has remained elusive. We are trying to borrow a page from Mother Nature whereby photosynthesis produces energy from the sun and stores it,” said lead author Dunwei Wang, an associate professor of chemistry at the school.
Energy production and storage “requires materials that can absorb sunlight broadly, transfer the energy to excited charges at high efficiencies and catalyze specific reduction and oxidation reactions,” according to the article.
Artificial photosynthesis, or water splitting, uses a photoanode to oxidize water and a photocathode to either reduce water for hydrogen production or to reduce carbon dioxide for organic molecules. These combined reactions require 1.2 to 1.3 volts to power this artificial approach to what trees and grass do naturally.
Rather than rely on the expensive materials previous researchers used for the redox reactions, Wang and his team coated hematite, an iron oxide similar to rust, with nickel iron oxide. They are still two-tenths of a volt from having the anode and cathode match required performance. “Our system, made of oxygen, silicon and iron – three of the four most abundant elements on earth – can now provide more than 1 volt of power together,” claims Wang.
The school reports, “Already, the team has yielded more than 1 volt of power when combined with the photocathode they developed earlier this year, said Wang, whose team included post-doctoral researcher Xiaogang Yang, graduate students Chun Du, Matthew T. Mayer and Jin Xie, undergraduates Henry Hoyt and Gregory Bischoping and Gregory McMahon, a nanolithography and electron microscopy manager at BC’s Nanofabrication Clean Room.”
Because they are so close to closing the voltage gap between anode and cathode, other teams are showing interest, the Boston College team might team up with other researchers to push toward a final success.
“’With our innovations on the photocathode alone, this two-tenths of a volt is within reach,’ said Wang. ‘The real exciting part is that we were able to achieve six tenths of a volt using rust. That has never been done before.’”
