Several sources report on Samsung’s announcement that they have developed a new technology that enables them to coat silicon battery cathodes with high crystal graphene, virtually doubling the capacity of lithium-ion batteries.
Of course, Samsung relates this immediately to their popular smartphones and tablets, but the significance of this is not lost on electric vehicle designers. Doubling the range of EVs “without adding a single pound of weight” would be a true game changer. But don’t get excited too quickly.
Silicon electrodes have been a major research effort for people like Dr. Yi Cui, who spoke at this year’s Electric Aircraft Symposium. Issue surrounding their successful use have included silicon’s expansion when being charged and contraction when being discharged. This errant flexibility causes eventual disintegration of the electrodes and shuts down the battery. Attempts to use silicon nanowires still have led to embrittlement.
Kompulsa.com reports Cho Jin-young from BusinessKorea explaining, “Currently, the development of high-capacity battery materials has been mostly done in the United States. In particular, the research is active on silicon as a substitute material capable of raising the capacity more than 10 times that of the graphite currently used as an existing cathode material. There is, however, still the technological problem of the shortening the battery life by repeated charging and discharging.”
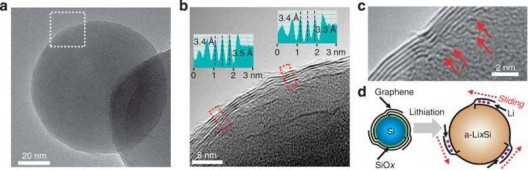
SiC-free graphene growth on Si NPs. (a) A low-magnification TEM image of Gr–Si NP. (b) A higher-magnification TEM image for the same Gr–Si NP from the white box in a. (Insets) The line profiles from the two red boxes indicate that the interlayer spacing between graphene layers is ~3.4 Å, in good agreement with that of typical graphene layers based on van der Waals interaction. (c) A high-magnification TEM image visualizing the origins (red arrows) from which individual graphene layers grow. (d) A schematic illustration showing the sliding process of the graphene coating layers that can buffer the volume expansion of Si. Credit: Nature Communications 6, Article number: 7393 doi:10.1038/ncomms8393
Potential energy densities of 1,000 Watt-hours per kilogram are a great lure for further research, opening possibilities of batteries with five times the energy density of currently-available lithium-ion competitors.
Phys.org reports, “To circumvent that problem, the researches grew carbide-free graphene (to keep it from forming they developed a chemical vapor deposition process which included using a mild oxidant) on its surface creating a protective and restrictive coating. In addition to preventing expansion, the graphene also helped prevent the silicon from breaking down over time (which occurs due to constant expanding and contracting).”
Not quite the double promised in the headlines, the test battery had an initial energy density 1.8 times that of conventional batteries, and held steady at 1.5 times after repeated use. Two things stand in the way of commercializing this breakthrough. Graphene is not easily made in large quantities – yet. And the laboratory techniques Samsung researchers used so brilliantly to coat silicon with graphene will need to be scaled up significantly to allow factory-level production.
We see loads of laboratory breakthroughs, but until someone comes up with ways to make these breakthroughs affordable and mass-producible, they are unfortunately still in the hoped-for near future. Let’s hope Samsung and others are intent on that since the first out of that gate could garner enormous profits.
Samsung and its academic partners published their findings in Nature Communications. The abstract provides a little more insight into the work and ends with some hope that this research will reach the market.
“Silicon is receiving discernable attention as an active material for next generation lithium-ion battery anodes because of its unparalleled gravimetric capacity. However, the large volume change of silicon over charge–discharge cycles weakens its competitiveness in the volumetric energy density and cycle life. Here we report direct graphene growth over silicon nanoparticles without silicon carbide formation. The graphene layers anchored onto the silicon surface accommodate the volume expansion of silicon via a sliding process between adjacent graphene layers. When paired with a commercial lithium cobalt oxide cathode, the silicon carbide-free graphene coating allows the full cell to reach volumetric energy densities of 972 and 700 Wh l−1 at first and 200th cycle, respectively, 1.8 and 1.5 times higher than those of current commercial lithium-ion batteries. This observation suggests that two-dimensional layered structure of graphene and its silicon carbide-free integration with silicon can serve as a prototype in advancing silicon anodes to commercially viable technology.”
