intercalation (countable and uncountable, plural intercalations)
Wiktionary
Enyuan Hu, a chemist at Brookhaven National Laboratory explains the importance, and limitations, of intercalation in battery chemistry. “The materials normally used in lithium-ion batteries are based on intercalation chemistry. This type of chemical reaction is very efficient; however, it only transfers a single electron, so the cathode capacity is limited. Some compounds like FeF3 are capable of transferring multiple electrons through a more complex reaction mechanism, called a conversion reaction.”
Iron trifluoride (FeF3) is composed of “cost-effective and environmentally benign elements — iron and fluorine. Researchers have been interested in using chemical compounds like FeF3 in lithium-ion batteries because they offer inherently higher capacities than traditional cathode materials,” according to Brookhaven.
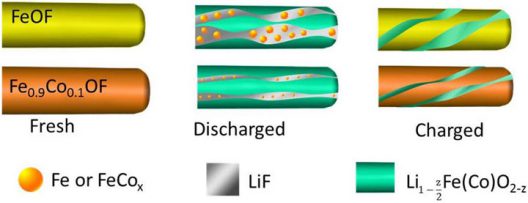
Substituting the cathode material with oxygen and cobalt prevents lithium from breaking chemical bonds and preserves the material’s structure
Scientists at the University of Maryland (which led the research), Brookhaven and the U.S. Army Research Lab developed and studied the FeF3 cathode. Xiulin Fan, a scientist at UMD and one of the lead authors of a paper appearing in Nature Communications, explains,” Cathode materials are always the bottleneck for further improving the energy density of lithium-ion batteries.”
Materials used in most battery cathodes allow the transfer of only one electron at a time, according to the researchers. UMD’s FeF3 enables tripling that transfer capability. It also helps overcome problems that handicapped earlier version of the material: “poor energy efficiency (hysteresis), a slow reaction rate, and side reactions that can cause poor cycling life.”
According to Brookhaven, “To overcome these challenges, the scientists added cobalt and oxygen atoms to FeF3 nanorods through a process called chemical substitution. This allowed the scientists to manipulate the reaction pathway and make it more ‘reversible.’”
Sooyeon Hwang, a co-author of the paper and a scientist at Brookhaven’s Center for Functional Nanomaterials (CFN), explained further. “When lithium ions are inserted into FeF3, the material is converted to iron and lithium fluoride. However, the reaction is not fully reversible. After substituting with cobalt and oxygen, the main framework of the cathode material is better maintained and the reaction becomes more reversible.”
Working at such nano levels requires high-powered equipment. Brookhaven has exotic research tools at the Center for Functional Nanomaterials (CFN), which explores the unique properties of materials and processes at the nanoscale. Brookhaven also features the National Synchrotron Light Source II (NSLS-II), a resource with “ultra-bright X-ray” equipment.

Brookhaven scientists Enyuan Hu and Sooyeon Hwang are pictured at the Center for Functional Nanomaterial’s TEM facility where the researchers viewed the cathode material at a resolution of 0.1 nanometers.
Researchers used transmission electron microscopy (TEM) at CFN to look at FeF3 nanorods at a resolution of 0.1 nanometers. This enabled them, “To determine the exact size of the nanoparticles in the cathode structure and analyze how the structure changed between different phases of the charge-discharge process.” They saw a faster reaction speed for the substituted materials.
Using NSLS-II’s X-ray Powder Diffraction (XPD) beamline, and directing ultra-bright x-rays through the cathode material, researchers were able to analyze light scattering to “see” additional information about the cathode’s structure.
Jianming Bai, a co-author of the paper and a scientist at NSLS-II explained, “The PDF analysis on the discharged cathodes clearly revealed that the chemical substitution promotes electrochemical reversibility.”
Xiao Ji, a scientist at UMD and co-author of the paper concluded, “Scientists at UMD say this research strategy could be applied to other high energy conversion materials, and future studies may use the approach to improve other battery systems.”
Investments in large-scale and undoubtedly expensive equipment will probably pay off in improved research techniques and much improved future batteries.

