The Earth we inhabit struggles in an ever-tightening race between being overcome with its own trash and cleaning up after itself. Two stunning approaches to turning trash and waste into valuable materials and fuels could help us win that race.
Graphene Dreams in a Flash
Bigthink.com leads with the news that, “Graphene typically costs $200,000 per ton. Now, scientists can make it from trash.” This “insanely useful” product is difficult to produce, making it a luxury for many applications – until now.
Graphene is insanely useful, but very difficult to produce — until now. Dr. James Tour and his Rice University students have created a way to produce graphene in large quantities in a literal flash. This technique, Flash Joule Heating, was discovered in Dr. Tour’s laboratory by graduate student Duy Luong. Also the lead author on the paper in the journal Nature, Luong “Did not expect to find graphene when he fired up the first small-scale device to find new phases of material, beginning with a sample of carbon black. ‘This started when I took a look at a Science paper talking about flash Joule heating to make phase-changing nanoparticles of metals,’ he said. But Luong quickly realized the process produced nothing but high-quality graphene.”
As Dr. Tour explains in the video below, Luong’s discovery came to have grander, even planet-saving implications.
world throws out 30% to 40% of all food, because it goes bad, and plastic waste is of worldwide concern. We’ve already proven that any solid carbon-based matter, including mixed plastic waste and rubber tires, can be turned into graphene.”
First, by turning just about any source of carbon from banana peels to plastic bottles into flash graphene, the world can save enormous amounts of energy otherwise spent hauling and dealing with trash. Dr. Tour explains, “This is a big deal. The second, by adding budget graphene to other products, large quantities of material that otherwise adds greenhouse gases to the atmosphere can be reduced and better final products achieved. For instance, “According to the most recent survey of Portland Cement Association (PCA) members, an average of 927 kg (2044 lb) of CO2 are emitted for every 1000 kg (2205 lb.) of Portland cement produced in the U.S.”
Rice University reports, “Tour said a concentration of as little as 0.1% of flash graphene in the cement used to bind concrete could lessen its massive environmental impact by a third. Production of cement reportedly emits as much as 8% of human-made carbon dioxide every year.
“By strengthening concrete with graphene, we could use less concrete for building, and it would cost less to manufacture and less to transport,” he said. “Essentially, we’re trapping greenhouse gases like carbon dioxide and methane that waste food would have emitted in landfills. We are converting those carbons into graphene and adding that graphene to concrete, thereby lowering the amount of carbon dioxide generated in concrete manufacture. It’s a win-win environmental scenario using graphene.”
Besides concrete, the flash graphene process can convert solid carbon into asphalt, buildings, cars, clothing and more, according to Dr. Tour. He sees it as a means to use coal to make clean graphene that will thus sequester an otherwise polluting fuel.
Graphene’s use in batteries has been explored and found to have possible benefits for energy and power density, and extending the lifespan of cells.
The paper’s abstract gives an overview of how Rice’s technology is unique. “Most bulk-scale graphene is produced by a top-down approach, exfoliating graphite, which often requires large amounts of solvent with high-energy mixing, shearing, sonication or electrochemical treatment1,2,3. Although chemical oxidation of graphite to graphene oxide promotes exfoliation, it requires harsh oxidants and leaves the graphene with a defective perforated structure after the subsequent reduction step3,4. Bottom-up synthesis of high-quality graphene is often restricted to ultrasmall amounts if performed by chemical vapor deposition or advanced synthetic organic methods, or it provides a defect-ridden structure if carried out in bulk solution4,5,6. Here we show that flash Joule heating of inexpensive carbon sources—such as coal, petroleum coke, biochar, carbon black, discarded food, rubber tires and mixed plastic waste—can afford gram-scale quantities of graphene in less than one second. The product, named flash graphene (FG) after the process used to produce it, shows turbostratic arrangement (that is, little order) between the stacked graphene layers. FG synthesis uses no furnace and no solvents or reactive gases. Yields depend on the carbon content of the source; when using a high-carbon source, such as carbon black, anthracitic coal or calcined coke, yields can range from 80 to 90 per cent with carbon purity greater than 99 per cent. No purification steps are necessary.”
Waste Plastics to Jet Fuel in an Hour
Washington State University researchers at the Pullman campus have converted plastics to jet fuel and “other valuable products” in an hour at moderate temperatures. Not quite as quick or “flashy” as the Rice breakthrough, WSU’s process has equally useful outcomes.
The school points out the over-arching threat plastics have to humanity. “In recent decades, the accumulation of waste plastics has caused an environmental crisis, polluting oceans and pristine environments around the world. As they degrade, tiny pieces of microplastics have been found to enter the food chain and become a potential, if unknown, threat to human health.” Even surfers organizing beach cleanup won’t necessarily make a sufficiently large dent in the garbage islands floating on our seas. But making usable products will help incentivize such efforts.
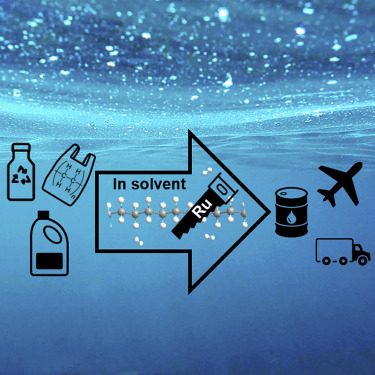
Graduate student Chuhua Jia and Hongfei Lin, associate professor in the Gene and Linda Voiland School of Chemical Engineering and Bioengineering, report on their work in the journal, Chem Catalysis. Their paper, “Deconstruction of high-density polyethylene into liquid hydrocarbon fuels and lubricants by hydrogenolysis over Ru catalyst” shows how low energy that deconstruction is. (Oversimplified, hydrogenolysis cuts the bonds holding the plastic together by exposure to hydrogen.)
Highlighting how important their efforts are toward meeting United Nations Sustainable Development Goals, the students point out that, “Collectively, ~6.3 billion metric tons of plastic waste were produced by 2015, of which 79% was landfilled, 12% was incinerated, and only 9% was recycled. PE is the polymer with the most massive volume produced globally, and the production could reach over 100 million metric tons per year. Therefore, the efficient upcycling of waste plastics, especially PE, is critical to mitigating the severe environmental problem.” Upcycling is an important concept here, since so much of plastic waste gets downcycled into products of lesser worth. Even for those things that have inherent value, how many park benches does the world need, anyway?
In a “milestone” that could “advance this new technology to commercialization,” researchers were able to convert 90 percent of their plastic feed stock to, Jet fuel and other valuable hydrocarbon products within an hour at moderate temperatures and to easily fine-tune the process to create the products that they want.” Low temperatures and flexibility of output will enable using these processes in other reactions.
Using a ruthenium on carbon catalyst and a commonly used solvent, researchers were able to convert about 90 percent of the plastic to jet fuel components or other hydrocarbon products within an hour at a temperature of 220 degrees Celsius (428 degrees Fahrenheit). There are no comments on how relatively clean the fuel obtained from the process will be compared to those derived directly from fossil sources.
“Adjusting processing conditions, such as the temperature, time or amount of catalyst used, provided the critically important step of being able to fine-tune the process to create desirable products.”
The team hopes to scale the process to industrial levels for commercialization. The work, which was done in collaboration with researchers from the University of Washington and Pacific Northwest National Laboratory, including Professor Jim Pfaendtner. It was funded by the Washington State Research Foundation and the National Science Foundation.