Mary Grady’s report at AvWeb alerted your editor to this exciting development.
Imagine a battery capable of seven times the energy output of any lithium battery now in existence, made of non-toxic, easily recycled materials. One aspect of this new energy source might give you pause, however. You have to set fire to the battery to extract all that energy.
With recalls of so-called “hoverboards” and still warm memories of Tesla and 787 Dreamliner battery fires, folks might be excused for wanting to avoid anything that combines fires with batteries. The new approach, from MIT researchers, uses carbon nanotubes as its base, and these don’t self-ignite like their lithium cousins.
Michael Strano, the Carbon P. Dubbs* Professor in Chemical Engineering at MIT found that coating a carbon nanotube with combustible material and lighting one end would produce a current as the fire progressed along the tube. Even though the amount of energy generated was low, Dr. Strano and his students persisted, increasing output and putting these flamers “in the same ballpark as today’s best batteries.” Strano adds, “” In fact, the energy efficiency is about 10,000 times greater than that reported in the original discovery paper. Even at that, the battery is only one percent efficient at converting heat to electricity.
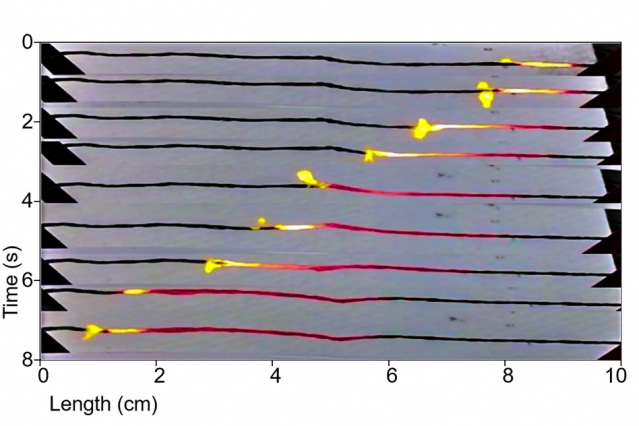
Thermopower waves slide along a series of carbon nanotubes. Clustering such tubes could provide for great bursts of power
As usual with such promising breakthroughs, the team suggests curbing our enthusiasm, a commercial products still being a few years away.
In the meantime, he provides a colorful way of envisioning the energy producing “wave” that travels along the carbon nanotubes, likening the energy being propagated to surfers riding that wave. Like surfers, the energy might “divide into two different components,” which can reinforce one another and sometimes counter each other.
Since the video was made six years ago, the researchers have allowed further revelations about their technology, sharing things like fuel sources. Notes accompanying the video show that “Electrical pulses of specific power as high as 7 kW kg-1were experimentally observed. This phenomenon of a self-propagating reaction wave leading to an electrical pulse generation was identified as thermopower waves. Studies show that the specific power scales inversely with the system size. This kind of energy source could have many potential applications because of its long shelf life and rapid energy discharge.”
Noting that lithium-ion technology has 25 years of development time, his nanotube technology has only about five years of experimentation so far. Since lithium is extremely flammable and bursts into flame in the open air, his battery, which uses carbon and sugar, is intrinsically safe in its makeup. Both ingredients are renewable, non-toxic and easily repurposed or recycled.
Better heat sources might generate more powerful waves, and the ability to store the dormant nanotube clusters and their fuel would enable their use on missions like long-term space flights. Scalability enables making this type of system large enough for grid power generation or wearable electronics devices.
Kourosh Kalantar-Zadeh, a professor of electrical and computer engineering at RMIT University in Australia, who was not involved in this research, says. “I believe that we are still far from the upper limit that the thermopower wave devices can potentially reach. However, this step makes the technology more attractive for real applications” partly because “We can obtain phenomenal bursts of power, which is not possible from batteries. For instance, the thermopower wave systems can be used for powering long-distance transmission units in micro- and nano-telecommunication hubs.”
The new results were published in the journal Energy & Environmental Science, in a paper by Strano, doctoral students Sayalee Mahajan PhD ’15 and Albert Liu, and five others.
The team also included Anton Cottrill, Yuichiro Kunai, David Bender, Javier Castillo Jr., and Stephen Gibbs. The work was supported by the Air Force Office of Scientific Research and the Office of Naval Research.
The paper’s abstract provides a few additional insights into the team’s efforts. “There is a pressing need to find alternatives to conventional batteries such as Li-ion, which contain toxic metals, present recycling difficulties due to harmful inorganic components, and rely on elements in finite global supply. Thermopower wave (TPW) devices, which convert chemical to electrical energy by means of self-propagating reaction waves guided along nanostructured thermal conduits, have the potential to address this demand. Herein, we demonstrate orders of magnitude higher chemical-to-electrical conversion efficiency of thermopower wave devices, in excess of 1%, with sustainable fuels such as sucrose and NaN3 for the first time, that produce energy densities on par with Li-ion batteries operating at 80% efficiency (0.2 MJ L−1 versus 0.8 MJ L−1). We show that efficiency can be increased significantly by selecting fuels such as sodium azide or sucrose with potassium nitrate to offset the inherent penalty in chemical potential imposed by strongly p-doping fuels, a validation of the predictions of Excess Thermopower theory. Such TPW devices can be scaled to lengths greater than 10 cm and durations longer than 10 s, an over 5-fold improvement over the highest reported values, and they are capable of powering a commercial LED device. Lastly, a mathematical model of wave propagation, coupling thermal and electron transport with energy losses, is presented to describe the dynamics of power generation, explaining why both unipolar and bipolar waveforms can be observed. These results represent a significant advancement toward realizing TPW devices as new portable, high power density energy sources that are metal-free.”
* Your editor was a bit non-plussed at the name, thinking it might be typo. Research revealed that there really was a Carbon Petroleum Dubbs, born in 1881, just as oil was coming into its own in his birth state of Pennsylvania. His father gave him the name “Carbon,” and he added the “P” when he was older to make the name “more euphonious.” People thought the middle initial might stand for “Petroleum” and the full name stuck.

