Going to medical school to learn how to use bacteria to make gasoline may seem like a complicated process, but the developers of a new way of extracting biofuels from sunlight say it’s not. You may remember Dr. Daniel Nocera’s efforts a few years ago to create a bionic leaf, a simple way to extract oxygen and hydrogen from water when the leaf in water was exposed to sunlight. Several other such “water splitters” have achieved newsworthiness in the last few years, but each has the impediment of not delivering hydrogen in a readily useable way.
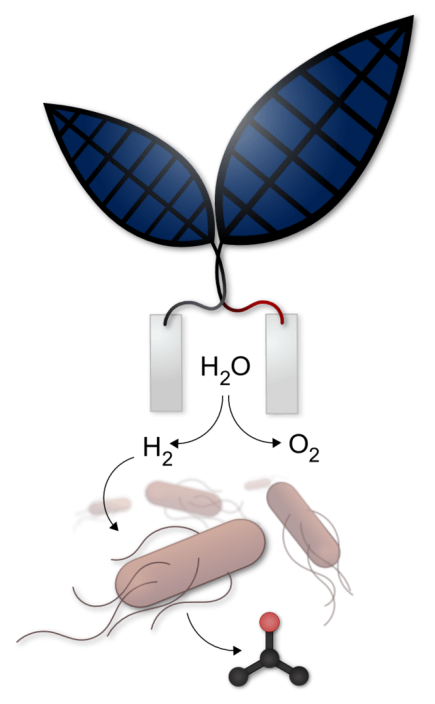
Usually, any H2 produced has to be compressed, stored in hydrides, or encapsulated in some way to make it a viable fuel. There is not a national infrastructure to allow hydrogen to be distributed as readily as gasoline or Diesel. Researchers working with Dr. Nocera “at Harvard University’s Faculty of Arts and Sciences, Harvard Medical School and the Wyss Institute for Biologically Inspired Engineering have created a system that uses bacteria to convert solar energy into a liquid fuel. Their work integrates an ‘artificial leaf,’ which uses a catalyst to make sunlight split water into hydrogen and oxygen, with a bacterium engineered to convert carbon dioxide plus hydrogen into the liquid fuel isopropanol.” Dr. Nocera is now the Patterson Rockwood Professor of Energy at Harvard.
The combination of organic (carbon-based) and inorganic chemistry seems to work some magic here, and helps one understand the need to bring in the med school as well as the chemists. Nocera called on the talents of Dr. Joseph Torella, a recent graduate from the Harvard Medical School Department of Systems Biology, and Christopher Gagliardi, a postdoctoral fellow in the Harvard Department of Chemistry and Chemical Biology.
Pamela Silver, the Elliott T. and Onie H. Adams Professor of Biochemistry and Systems Biology at HMS, terms the system a bionic leaf, referencing Dr. Nocera’s earlier artificial leaf.
When Nocera came to Harvard from MIT, he and Silver shared their interest in “personalized energy,” making energy production as localized as possible, rather than relying on the bigger centralized refining and distribution system with which we are all familiar.
Silver explains, “This is a proof of concept that you can have a way of harvesting solar energy and storing it in the form of a liquid fuel. Dan’s formidable discovery of the catalyst really set this off, and we had a mission of wanting to interface some kinds of organisms with the harvesting of solar energy. It was a perfect match.” She points out that localized energy could be attractive in the developing world. “It’s not like we’re trying to make some super-convoluted system. Instead, we are looking for simplicity and ease of use.”
Scientific American explains the process this way: …”Starve a microbe nearly to death, then feed it carbon dioxide and hydrogen produced with the help of voltage from a solar panel. A newly developed bioreactor feeds microbes with hydrogen from water split by special catalysts connected in a circuit with photovoltaics. Such a battery-like system may beat either purely biological or purely technological systems at turning sunlight into fuels and other useful molecules, the researchers now claim.”
Nocera’s original artificial leaf used simple, readily attainable materials to split water, as reported in this blog in 2012. “The ‘leaf’ is made of inexpensive materials bound onto a sheet of silicon. One side has a layer of a cobalt-based catalyst that releases oxygen and the other side has a layer of a nickel-molybdenum-zinc alloy, which release hydrogen. The device seems to be long-lasting and maintenance free, and can be used in even dirty water, often a given in poor countries.”
Nocera has held to the original design philosophy for the base leaf, saying, “The catalysts I made are extremely well adapted and compatible with the growth conditions you need for living organisms like a bacterium.”
Once the artificial leaf splits water into oxygen and hydrogen, the hydrogen is fed to a genetically engineered variant of Ralstonia eutropha, from which the research team made isopropanol (C3H8O), “an alcohol molecule that can be used as fuel like ethanol or gasoline and can be easily separated from water with salt.”
This system gets more bang for the buck than natural photosynthesis, beating nature’s rate of one percent efficiency in turning sunlight into biomass. Researchers are hoping to achieve five percent efficiency.
Brenda Colón, a graduate student in Silver’s lab and co-author or the team’s paper in the February 9 Proceedings of the National Academy of Sciences. looks at further developments. “The advantage of interfacing the inorganic catalyst with biology is you have an unprecedented platform for chemical synthesis that you don’t have with inorganic catalysts alone. Solar-to-chemical production is the heart of this paper, and so far we’ve been using plants for that, but we are using the unprecedented ability of biology to make lots of compounds.” Compounds could include drugs such as vitamins in small amounts, according to Silver. Other authors include Janice S. Chena, D. Kwabena Bediakob, and Jeffery C. Way.
In a not vainglorious statement, Nocera took pride in the team’s accomplishments. “We’re almost at a 1 percent efficiency rate of converting sunlight into isopropanol,” Nocera said. “There have been 2.6 billion years of evolution, and Pam and I working together a year and a half have already achieved the efficiency of photosynthesis.”
According to the abstract for their paper, they also set records for “engineering of R. eutropha enabled production of the fusel alcohol isopropanol at up to 216 mg/L, the highest bioelectrochemical fuel yield yet reported by >300%.”
Harvard reports, “This work was supported by Air Force Office of Scientific Research Grant FA9550-09-1-0689, Office of Naval Research Multidisciplinary University Research Initiative Award N00014-11-1-0725 and a National Science Foundation Graduate Research Fellowship.”