We tend to think of batteries as being inanimate objects, even though they expand, contract and flex their electric muscles within their cylindrical or pouch forms as they charge and discharge. This type of internal wiggling helps reduce and finally destroy the battery’s ability to make our remotes change channels or keep our airplanes flying.
Researchers at the Beijing Institute of Technology have found a way to use the product of much internal and external wiggling, natural silk that is “biomass-derived” and processed to form carbon-based nanosheets that might be used in lithium-ion batteries and other energy storage devices.
The American Chemical Society reports that Chuanbao Cao and his researchers worked with the idea that carbon is a key component in commercial Li-ion energy storage devices including batteries and supercapacitors. They wanted to find a natural and sustainable alternative to graphite, which has limited specific energy and eventually granulates into a fine powder, causing the battery to fail.
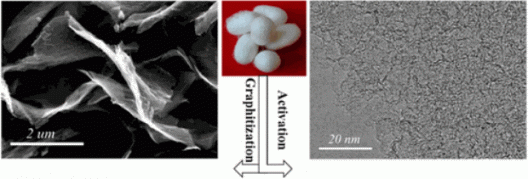
Cao and colleagues performed “simultaneous activation and graphitization of the silk,” making the processed silk into “hierarchical porous nitrogen-doped carbon (HPNC) nanosheets (NS).”
They dissolved the silk in iron chloride (FeCL3) and zinc chloride (ZnCl2), which acted “as effective activation-graphitization agents that can introduce a porous structure with plentiful micro- and mesopores for a high surface area.”
These sheets have a high specific surface area, covering an SBET of 2,494 square meters (26,845 square feet) per gram, or about 90-percent of a football field. “SBET,” stands for surface area as measured by a technique developed in 1938 by Stephen Brunauer, P.H. Emmet and Edward Teller to measure the specific surface of finely divided and porous solids.
The sheets self-assemble in hydrophilic (water loving) and hydrophobic (water hating) blocks in an aqueous system. This forms a series of different sheets which, because of their uneven surfaces, don’t quite come together and provide spaces between the sheets and through the many pores in their surfaces, for electrolyte and ions to flow.
As explained in Green Car Congress, “The resulting HPNC-NSs have a thickness in the range of 15 to 30 nm; the folds of the nanosheets limit them from stacking together. The layer-to-layer distance is 0.40 nm. The nanosheet architecture not only offers minimum diffusive resistance to mass transport on a large electrode/electrolytes interface for charge-transfer reaction but also provides easy ion transport by shortening the diffusion pathways.”
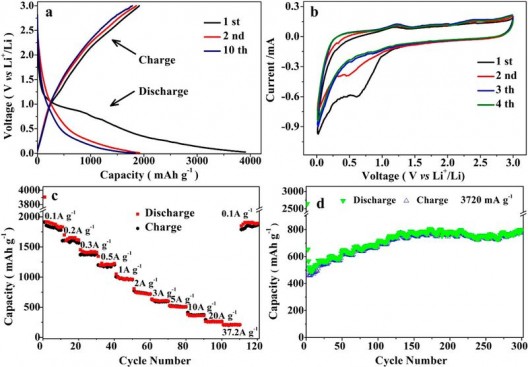
Electrochemical performance of HPNC-NS as a Li-ion battery anode. (a) charge-discharge curves at 0.1 Amp per gram: (b) CVs of initial four cycles at a scan rate of 0.1 millivolt per second; (c) capacity over cyclclingat different rates; and (d) cyclability at 3,270 mA/g. Credit: ACS, Hou et al.
The layers and pores have demonstrated excellent energy storage capacity and longevity, with the team reporting, “A reversible lithium storage capacity of 1865 mAh/g—the highest for N-doped carbon anode materials to the best of the researchers’ knowledge. Used as a supercapacitor electrode in ionic liquid electrolytes, the HPNC-NS exhibit a capacitance of 242 F/g and energy density of 102 Wh/kg (48 Wh/L), with high cycling life stability (9% loss after 10,000 cycles).”
Since the new materials seem to function well in supercapacitors and as battery anodes, and can be scaled for industrial production, there would seem to be a bright future for silkworms and their products. Will competition for silk cause clothing prices to rise, much like ethanol caused spikes in corn prices?
A paper, “Hierarchical Porous Nitrogen-Doped Carbon Nanosheets Derived from Silk for Ultrahigh-Capacity Battery Anodes and Supercapacitors,” describing the research was published in the journal ACS Nano.
The abstract gives an overview of the research and its results.
“Hierarchical porous nitrogen-doped carbon (HPNC) nanosheets (NS) have been prepared viasimultaneous activation and graphitization of biomass-derived natural silk. The as-obtained HPNC-NS show favorable features for electrochemical energy storage such as high specific surface area (SBET: 2494 m2/g), high volume of hierarchical pores (2.28 cm3/g), nanosheet structures, rich N-doping (4.7%), and defects. With respect to the multiple synergistic effects of these features, a lithium-ion battery anode and a two-electrode-based supercapacitor have been prepared. A reversible lithium storage capacity of 1865 mA h/g has been reported, which is the highest for N-doped carbon anode materials to the best of our knowledge. The HPNC-NS supercapacitor’s electrode in ionic liquid electrolytes exhibit a capacitance of 242 F/g and energy density of 102 W h/kg (48 W h/L), with high cycling life stability (9% loss after 10 000 cycles). Thus, a high-performance Li-ion battery and supercapacitors were successfully assembled for the same electrode material, which was obtained through a one-step and facile large-scale synthesis route. It is promising for next-generation hybrid energy storage and renewable delivery devices.”