The CAFE Blog has been tracking developments in batteries and supercapacitors for nearly the last two years, and the annual Electric Aircraft Symposia have attracted speakers on a wide range of innovations in these areas. Gizmag reminded us this week how much all of this may soon affect the ability of energy storage and power devices to change our world.
Their report highlighted work on an energy storage system that combines the energy capacity of batteries with the power density and quick recharging capabilities of capacitors being done at Rice University, and linked that to research at the University of Illinois by Dr. Paul Braun on creating faster charging batteries with higher energy densities than currently possible. Gizmag then did a callback to something covered earlier by CAFE, the use of structural panels in electric vehicles as energy storage devices.
Both researchers pointed to the big disappointment in electrical devices – batteries. They are either short-lived in their application or take too long to recharge. Researchers at both schools each took different approaches to overcoming at least one cause for battery buyers’ regret.
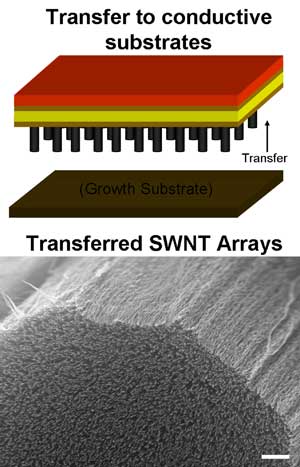
Not surprisingly, Rice’s research team, headed by Robert Hauge, Distinguished Faculty Fellow in Chemistry, used nanotechnology to gain the added surface area for their electrode assembly. Arranging hollow carbon nanotubes into a neat array of parallel shafts enclosed between two substrates requires great precision, but yields significant results.
Rice’s press release explains the constraints of “traditional” supercapacitors. “Electric double-layer capacitors (EDLCs), generally known as supercapacitors, solve the problems of both to some degree. They’re hybrids that hold hundreds of times more energy than a standard capacitor while retaining their fast charge/discharge capabilities. But traditional EDLCs rely on liquid or gel-like electrolytes that can break down in extreme conditions.”
Hauge’s researchers got rid of the electrolyte, replacing it with a nanoscale solid oxide dielectric coating that takes advantage of the extra surface area provided by the carbon nanotube arrays, which look like a “microscopic shag carpet.” Hundreds of thousands of these 50-micron long arrays may be placed on a single microchip, making for a powerful micro device. Graduate students Cary Pint and Nolan Nicholas worked with Hauge on this process, and are both now researchers for major electronics firms. This is significant because it spreads the technical expertise into areas which often have greater funding capacities than cash-strapped educational institutions.
The Rice press release continues. “The array was then transferred to a copper electrode with thin layers of gold and titanium to aid adhesion and electrical stability. The nanotube bundles (the primary electrodes) were doped with sulfuric acid to enhance their conductive properties; then they were covered with thin coats of aluminum oxide (the dielectric layer) and aluminum-doped zinc oxide (the counterelectrode) through a process called atomic layer deposition (ALD). A top electrode of silver paint completed the circuit.
“’Essentially, you get this metal/insulator/metal structure,’ Pint said. ‘No one’s ever done this with such a high-aspect-ratio material and utilizing a process like ALD.’”
Hauge noted the scalability of the process, and Pint explained the solid state supercapacitor could be integrated into different materials, as large as an electric car body or as small as a microbot that could be injected into a patient’s blood stream. This structure as body idea was posited by London’s Imperial College and reported here last year.
Paul Braun, a professor of materials science and engineering at the University of Illinois, Urbana-Champaign has taken a different approach to getting capacitor-like recharging speed and battery energy density in the same product.
He and his co-researchers, Huigang Zhang and Xindi Yu, published their findings in Nature Nanotechnology under the title, “Three-dimensional bicontinuous ultrafast-charge and -discharge bulk battery electrodes.”
The paper’s abstract notes the need for rapid charge and discharge rates for energy storage devices, and explains that although capacitors have that capability, they lack the necessary capacity to store
energy over time.
Their process creates a device combining the best of both. ”A storage technology that combines the rate performance of supercapacitors with the energy density of batteries would significantly advance portable and distributed power technology. Here, we demonstrate very large battery charge and discharge rates with minimal capacity loss by using cathodes made from a self-assembled three-dimensional bicontinuous nanoarchitecture consisting of an electrolytically active material sandwiched between rapid ion and electron transport pathways. Rates of up to 400C and 1,000C for lithium-ion and nickel-metal hydride chemistries, respectively, are achieved (where a 1C rate represents a one-hour complete charge or discharge), enabling fabrication of a lithium-ion battery that can be 90% charged in 2 minutes.”
Model airplane builders sometimes approach 40-50 C, but risk immolating their craft as a result of lithium fires. This rather incredible charge/discharge capability (assuming safety is retained) would mean great acceleration and instant power for vehicles while dyill storing energy for long endurance runs.
If either of these approaches provides a lightweight, energy-dense structural system, we could see a revolution in not only transportation, but in medicine, communication, and other areas requiring portable power.

Comments 1
I proposed the battery as core concept in 2008 and got a very negative response. Funny how that worked out.
Happy landings