A “novel” manganese and sodium-ion-based material might be a contender for the more universal lithium-ion batteries that power our mobile devices and make us mobile in electric vehicles. Developed by the University of Texas at Dallas in collaboration with Seoul National University, the new material is said to offer “a potentially lower-cost, more ecofriendly option to fuel next-generation devices and electric cars.”
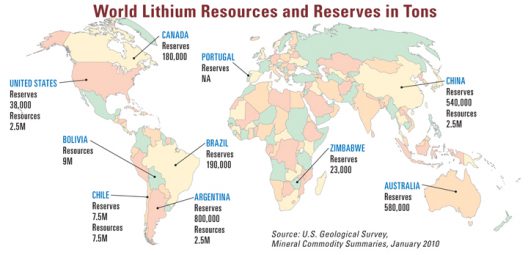
Worldwide distribution of lithium deposits, often in countries at odds with US interests
Dr. Kyeongjae Cho, professor of materials science and engineering in the Erik Jonsson School of Engineering and Computer Science, thinks battery cost is a “substantial issue.” It may become more of one with electric vehicle production growing from the existing global electric car stock of two million vehicles in 2016 to a projected nine-to-20 million (as reported by the International Energy Agency) by 2020 and between 40 and 70 million by 2025. This rapid growth will put an added burden on finding and extracting lithium, since the mineral exists in relatively small amounts in sometimes isolated places.
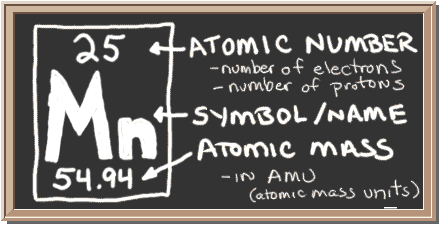
Correction added July 21: More common, “Manganese is a chemical element with symbol Mn and atomic number 25. It is not found as a free element in nature; it is often found in minerals in combination with iron. Manganese is a metal with important industrial metal alloy uses, particularly in stainless steels.” (Wikipedia, which would have corrected your editor just as alert reader Howard Handelman did:
Dr. Cho takes an Edison-like approach to sorting out a solution to this shortcoming. He questioned, “We used our previous experience and thought about these issues — how can we combine these ideas to come up with something new to solve the problem?”
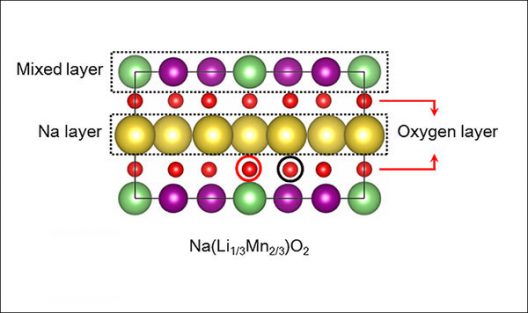
The research team’s sodium-ion design, which retains the high energy density of a lithium-ion cathode, replaces most of the lithium atoms (green) with sodium (yellow). The layered structure of the new material also incorporates manganese (purple) and oxygen (red).
The research team explains business as usual in a conventional lithium-ion cell: “A battery consists of a positive electrode or cathode; a negative electrode, or anode; and an electrolyte in between. In a standard lithium-ion battery, the cathode is made of lithium, cobalt, nickel, and oxygen, while the anode is made of graphite, a type of carbon. When the battery charges, lithium ions move through the electrolyte to the anode and attach to the carbon. During discharge, the lithium ions move back to the cathode and provide electric energy to run devices.”
Dr. Cho noted the difficulty in previous attempts to substitute manganese oxide in such batteries. “There was great hope several years ago in using manganese oxide in lithium-ion battery cathodes to increase capacity, but unfortunately, that combination becomes unstable.”
In the team’s new design, sodium replaces most of the lithium in the cathode and manganese replaces the more expensive cobalt and nickel. Dr. Cho thinks the sodium-ion material is more stable than lithium but retains its high energy capacity. He believes it’s scalable, enabling its use in commercial-sized production.
The team used “rational material design” for their studies, turning first to computer simulations to find promising atom configurations before making and testing the material in the laboratory. Dr. Cho thinks the approach is as important as the material.
“When Thomas Edison was trying to develop a light bulb, he tried thousands of different materials for the filament to see which ones worked. To solve very important engineering problems in society today, we need to develop lots of new materials — battery materials, pollution control materials and others. Edison was perfecting one item — the light bulb — but we have so many more technological needs. We don’t have time to keep trying to accidentally find the solution.”
The team published a paper about the new material in the journal Advanced Materials.
Co-authors of the study are lead author and graduate student Duho Kim and Dr. Maenghyo Cho, both at Seoul National University in Korea. The research was funded by the National Research Foundation of Korea and the Ministry of Trade, Industry and Energy of Korea.
Certainly a battery that is cheaper is desirable, but it remains to be seen whether the 20-percent differential in energy density can be overcome. As always, further research will help sort that out.