Dr. Yi Cui seems to get inspiration from food. A few years ago, his research team came up with a “yolk-shell structure” that helped contain the high amount of lithium that silicon anodes were able to absorb. That battery design promised much, and an embellishment of that design seems to hold even greater promise.
His newest effort, working at Stanford University with the Department of Energy’s SLAC National Accelerator Laboratory, features an electrode “designed like a pomegranate – with silicon nanoparticles clustered like seed in a tough carbon rind.” This approach, according to its inventors, overcomes several remaining obstacles to the use of silicon in a new generation of lithium-ion batteries.
Yi said the battery’s efficiency and longevity are promising. “Experiments showed our pomegranate-inspired anode operates at 97 percent capacity even after 1,000 cycles of charging and discharging, which puts it well within the desired range for commercial operation.”
Cui’s team has been working on preventing anode breakup for the last eight years, exploring how to reduce silicon to such small pieces that can’t be broken any farther when being charged. The silicon nanoparticles are then encased in carbon “yolk shells,” emulating the original research model. The carbon shell allows swelling and shrinking without breakage and prevents the buildup of “gunk” resulting from such breakdowns. This silicon-lithium gunk prevents efficient transfer of ions and reduces battery performance.
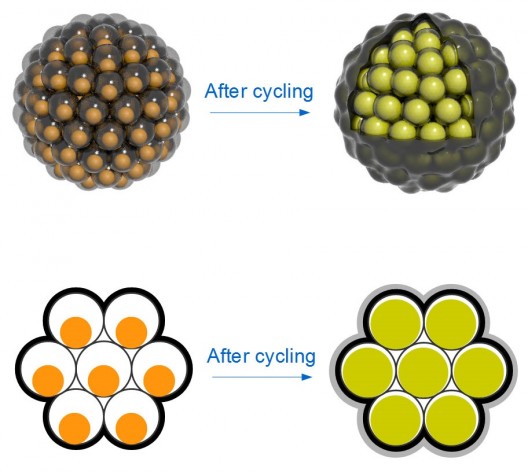
Top: Silicon nanoparticles are encased in carbon “yolk shells” and clustered like seeds in a pomegranate. Each cluster has a carbon rind that holds it together, conducts electricity and minimizes reactions with the battery’s electrolyte that can degrade performance. Bottom: Silicon nanoparticles swell during battery charging to completely fill their yolk shells; no space is wasted, and the shells stay intact. (Nian Liu, Zhenda Lu and Yi Cui/Stanford)
According to SLAC, “Graduate student Nian Liu and postdoctoral researcher Zhenda Lu used a microemulsion technique common in the oil, paint and cosmetic industries to gather silicon yolk shells into clusters, and coated each cluster with a second, thicker layer of carbon. These carbon rinds hold the pomegranate clusters together and provide a sturdy highway for electrical currents.”
Each pomegranate cluster has just one-tenth the surface area of the individual particles inside it, exposing a small surface area to the electrolyte, “thereby reducing the amount of gunk that forms to a manageable level.”
These almost sub-microscopic particles appear as a fine black powder rather than the more identifiable artist’s concept, but they are used to coat a piece of foil and form and anode. Tests showed the pomegranate anodes worked well in “the thickness required for commercial battery performance.”
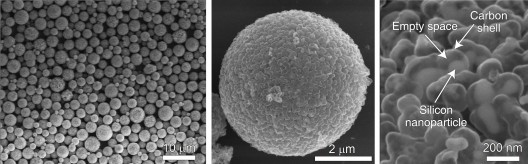
By precisely controlling the process used to make them, Stanford and SLAC researchers can produce pomegranate clusters of a specific size for silicon battery anodes. Left: Microscopic clusters form a fine black powder that can be coated on foil to create an anode. Middle: A single cluster. Right: In this close-up of a cluster, a silicon nanoparticle can be seen inside its yolk shell, with space to swell during battery charging. (Nian Liu, Zhenda Lu and Yi Cui/Stanford)
To make these breakthroughs commercially viable, Dr. Cui and his team need to simplify the process and find a “cheaper source of silicon nanoparticles.” Keeping with the food inspiration for the project so far, they may use rice husks – unfit for human consumption, produced by the millions of tons, and 20 percent silicon dioxide by weight. Liu reported on the ease of converting rice husks into pure silicon nanoparticles in a recent edition of Scientific Reports.
The battery is reported have “remarkable performance.” Green Car Congress reports that its reversible capacity reached 2,350 mAh g-1 for a rate of c/20 (1C ¼ charge/discharge in 1 hour). 2,350 Amp-hours per kilogram would be about 10 times the current norm for the best lithium-ion batteries. Volumetric efficiency is more than twice the 600 mAh cm-3 obtained for graphite anodes.
Icing on the pomegranate cake, capacity retention suggests a long life cycle for this type of battery. “From the 2nd to 1,000th cycle at a rate of C/2, the capacity retention was more than 97%. After 1,000 cycles, over 1,160 mAhg-1 capacity remained, which is more than three times the theoretical capacity of graphite. The cycle stability (0.003% decay per cycle) is among the best cycling performances of silicon anodes reported to date.”
Coulombic efficiency, the ability to hold a charge was as high as 99.87 percent from the 500th to the 1,000th cycles, “at a relatively slow rate of C/2.”
“To me it’s very exciting to see how much progress we’ve made in the last seven or eight years,” Cui said, “and how we have solved the problems one by one.”
The research team also included Jie Zhao, Matthew T. McDowell, Hyun-Wook Lee and Wenting Zhao of Stanford. Cui is a member of the Stanford Institute for Materials and Energy Sciences, a joint SLAC/Stanford institute. The research was funded by the DOE Office of Energy Efficiency and Renewable Energy.
The team’s findings are published in the journal Nature Nanotechnology.

