Replacing the graphite used in conventional battery electrodes with “a network of tin-oxide nanoparticles” could reduce battery charging time from hours to minutes. An energy storage device combining the advantages of batteries and capacitors is a long-term goal for researchers, and a multi-national discovery may help expedite that goal.
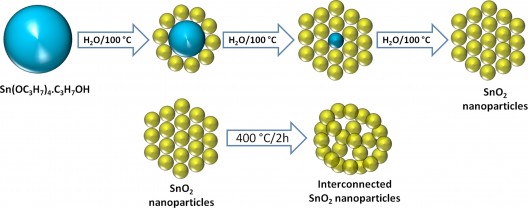
This schematic diagram depicts the concept for a new electrode design for lithium-ion batteries that has been shown to potentially reduce the charging time from hours to minutes by replacing the conventional graphite electrode with a network of tin-oxide nanoparticles. (Purdue University image/Vinodkumar Etacheri)
Graphite anodes and cathodes, as used in most lithium batteries today, limit storage capacities to 372 milliampere hours per gram (mA·h/g), the theoretical maximum of graphite. By comparison, an Energizer Ultimate Lithium AA battery holds about 3,000 mAh and weighs 14.5 grams (or about 207 mA h/g). A typical rechargeable AA battery holds only 750 to 900 mAh (around 54 to 64 mA h/g). This limit “hinders significant advances in battery technology,” according to Vilas Pol, Associate Professor of Chemical Engineering at Purdue University.
There, Pol, postdoctoral research associate Vinodkumar Etacheri, and other researchers internationally have experimented with a “porous interconnected” tin-oxide-based anode, giving twice the theoretical charging capacity of graphite. Not quite capacitor-quick, but speedier than normal slow charging, the anode can be charged in 30 minutes as opposed to a slow charge of 10 hours for the graphite anode. The experimental tin-oxide anode has a capacity of 430 mA h/g. Undoubtedly, this capacity will be restricted by electrolytes or other components of lesser capacity. The trick still seems to be to develop a wholistic battery approach, making an integrated system that optimizes the performance of all components.
The anode’s “ordered network” of tin oxide nanoparticles has commercial promise, being “synethsized by adding the tin alkoxide precursor into boiling water followed by heat treatment,” according to Pol.
Pol explains, “We are not using any sophisticated chemistry here. This is very straightforward rapid ‘cooking’ of a metal-organic precursor in boiling water. The precursor compound is a solid tin alkoxide—a material analogous to cost-efficient and broadly available titanium alkoxides. It will certainly become fully affordable in the perspective of broad-scale applications.”
Heating the tin oxide nanoparticles at 400°C causes them to self-assemble into a network containing pores that allow the material to expand and contract, or breathe, during the charge-discharge battery cycle.
While other electrode researchers have tried constraining their materials to prevent expansion and contraction, Vinodkumar Etacheri explains that, “These spaces are very important for this architecture, Without the proper pore size, and interconnection between individual tin oxide nanoparticles, the battery fails.”
The group’s finding are published in the November issue of the journal Advanced Energy Materials.
According to the Purdue press release, the research paper was authored by Etacheri; Swedish University of Agricultural Sciences researchers Gulaim A. Seisenbaeva, Geoffrey Daniel and Vadim G. Kessler; James Caruthers, Purdue’s Gerald and Sarah Skidmore Professor of Chemical Engineering; Jeàn-Marie Nedelec, a researcher from Clermont Université in France; and Pol.
Electron microscopy studies were performed at the Birck Nanotechnology Center in Purdue’s Discovery Park. Future research will include work to test the battery’s ability to operate over many charge-discharge cycles in fully functioning batteries.
