Graphene is science fiction made real – a one-atom thick layer of hexagonal arrays of carbon which weigh next to nothing and are stronger than any other material on earth. Wrap a layer of this stuff around “a novel multifunctional sulfur electrode that combines an energy storage unit and electron/ion transfer networks,” and you get “an extremely promising electrode structure design for rechargeable lithium-sulfur batteries.”
Lithium-sulfur batteries have the promise of reaching a theoretical specific energy density “approaching 2,600 Watt-hours per kilogram (Wh kg-1),” compared to currently available specific energy densities for lithium-ion cells of 130-220 Wh kg-1.
Researchers led by Dr. Vasant Kumar at the University of Cambridge and Professor Renjie Chen at the Beijing Institute of Technology worked to overcome the shortcomings of lithium-sulfur batteries now under development – a “fading” of the sulfur through a series of reactions with the anode, electrolyte, and lithium cathode, a kind of “shuttling” between these battery components, and the small number of charge-discharge cycles most such cells achieve.
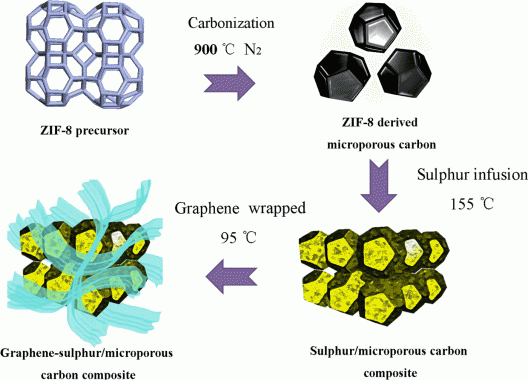
In a wildly oversimplified explanation, crafting a nano-scale Zeolitic Imidazolate Framework-8 (ZIF-8) and carbonizing it at 900° C (1,652° F) in a nitrogen (N2) atmosphere gave the researchers a ZIF-8 microporous carbon block, much like charcoal, with porous surfaces into which sulfur could be infused at 155° C (311° F). This formed a sulfur/microporous carbon composite around which to wrap graphene at 95° C (203° F), ending with a graphene sulfur/microporous carbon composite.
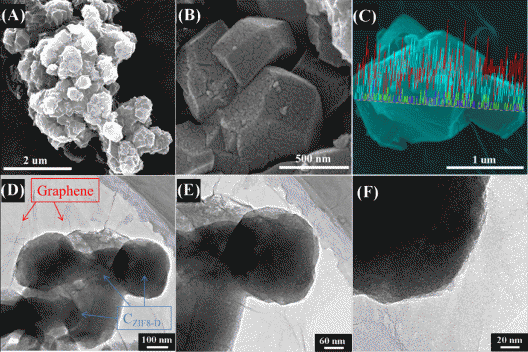
(a)–(c) Scanning Electron Microscope (SEM) images of the S/C ZIF8-D composite; (d)–(f) Transmission Electron Microscopy (TEM) images of the GS-S/C ZIF8-D composite
The ZIF-8 is a type of metal organic framework (MOF) that can be used for hydrogen storage, carbon dioxide sequestration, catalysis and membranes for various applications. Carbonized and wrapped in graphene, the new material is a conductive porous carbon cage, in which the infused sulfur acts as the host and “each sulfur carbon nanoparticle acts as [an energy storage unit] in which electrochemical reactions occur.” This wrapping speeds up the transfer of electrons and ions, and the fissures within the charcoal-like interior act as relatively voluminous energy storage system. There’s apparently a synergy between the parts of the total electrode that gives higher performance for the entirety than would be promised by its parts. Some of this comes from the graphene coating acting as a “bridge… to form inter-connected networks and thus reduc[e] the internal resistances from each component.”
What’s next for the team? “We’ll focus on fabricating hybrid free-standing sulfur cathode systems to achieve high-energy density batteries, which will involve tailoring novel electrolyte components and building lithium ‘protection layers’ to enhance the electrochemical performance of batteries,” explains research association Kian Xi.
Their article, “Graphene-wrapped sulfur/metal organic framework (MOF)-derived microporous carbon composite for lithium sulfur batteries,” is authored by Renjie Chen, Teng Zhao, Tian Tian, Shuai Cao, Paul R. Coxon, Kai Xi, David Fairen-Jimenez, R. Vasant Kumar and Anthony K. Cheetham. Appears in the journal APL Materials.