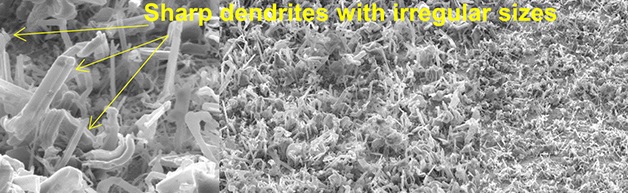
Hanqing Jiang, a professor in ASU’s School for Engineering of Matter, Transport and Energy, has come up with a clever and inexpensive way to fight dendrites in lithium batteries. Since these spiky little outbreaks can lead to battery fires, his team’s findings might lead to safer batteries. The approach involves silicone.
Many of us put up a (usually futile) fight against wrinkles, our youth culture spending fortunes to avoid the inevitable. Scientists at Arizona State University, however, are encouraging wrinkles in their lithium-metal batteries, and pouring cheap silicone goo over their anodes to discourage dendrites from popping up.
This novel approach to crafting lithium metal anodes for batteries is something Arizona State University scientists are working on, with surprising results. Hanqing Jiang, a professor in ASU’s School for Engineering of Matter, Transport and Energy, in the Ira A. Fulton Schools of Engineering
Silicon or Silicone?
Live Science explains an important distinction. “In short, silicon is a naturally occurring chemical element, whereas silicone is a synthetic substance.
“Silicon is the 14th element on the periodic table. It’s a metalloid, meaning it has properties of both metals and nonmetals, and is the second most abundant element in the Earth’s crust, after oxygen.
“Silicon readily bonds with oxygen and is rarely found in nature in its pure form. You’ve likely seen silicon as silicon dioxide or silica, better known as quartz, which is the most common component of sand.”
That it also makes up a major part of glass and computer chips shows the versatility of this common element.
Silicone, on the other hand, combines silicon with oxygen, carbon and hydrogen to make “generally a liquid or… flexible, rubberlike plastic” we use to calk our windows or enhance our trophy wives.
Reducing Stress
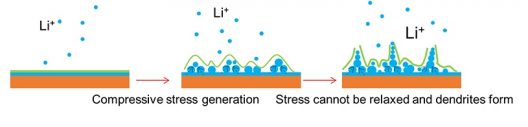
When lithium metal is deposited onto a rigid surface (the orange surface above), compressive stresses are formed, which cannot be relaxed and dendrites form (Image source: Arizona State University)
Dr. Jiang’s research at ASU may help lithium metal anodes keep their cool in batteries while doubling the energy storage capacity over carbon-based anodes used in many (most?) of today’s lithium ion cells.
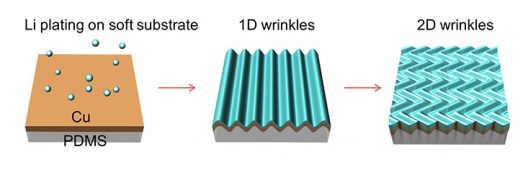
Plating of the lithium metal onto the silicone (PDMS) substrate causes it to wrinkle in 2 dimensions, reducing the lithium metal residual stress and dendrite formation (Image source: Arizona State University)
Beginning by depositing a layer of lithium metal onto a soft substrate of polydimethylsiloxane (PDMS or silicone) researchers then observed wrinkles forming in the silicone. According to ASU, “When the lithium metal was deposited on the silicone substrate, the stresses created by the accumulation of the metal were relieved by the formation of wrinkles in the silicon substrate. The elimination of the residual stresses had a large effect on the dendrites. ‘There were remarkable reductions in dendrite growth,’ said Jiang. The research team discovered that the reduction in dendrite growth was directly related to the reduction in stress caused by the deformation and wrinkling of the silicon substrate.”
Dr. Jiang explained the significance of the reaction. “We already know that tiny tin needles or whiskers can protrude out of tin surfaces under stress, so by analogy we looked at the possibility of stress as a factor in lithium dendrite growth.”
3D Equals Longer Life
The team found that giving the silicone a three-dimensional form, “almost like a sponge,” relieved stress and effectively inhibited dendrite growth. Jiang compares the form to a sugar cube, with PDMS forming a continuous network as the substrate covered by a thin copper layer to conduct electrons. Lithium fills these pores.
Zinc, sodium, and aluminum batteries have the same tendency to form dendrites, so the use of silicone could help develop safe, high-energy density batteries with several other metals .
The Team’s Paper
Xu Wang, Wei Zeng, Liang Hong, Wenwen Xu, Haokai Yang, Fan Wang, Huigao Duan, Ming Tang and Hanqing Jiang participated in the research. Their paper, “Stress-driven lithium dendrite growth mechanism and dendrite mitigation by electroplating on soft substrates,” appeared in the March 6, 2018 issue of Nature Energy.
The abstract for the team’s paper gives an introduction to their research and hints that their approach results in enhanced performance and great longevity for batteries using their technique.
“Problems related to dendrite growth on lithium-metal anodes such as capacity loss and short circuit present major barriers to next-generation high-energy-density batteries. The development of successful lithium dendrite mitigation strategies is impeded by an incomplete understanding of the Li dendrite growth mechanisms, and in particular, Li-plating-induced internal stress in Li metal and its effect on Li growth morphology are not well addressed. Here, we reveal the enabling role of plating residual stress in dendrite formation through depositing Li on soft substrates and a stress-driven dendrite growth model. We show that dendrite growth is mitigated on such soft substrates through surface-wrinkling-induced stress relaxation in the deposited Li film. We demonstrate that this dendrite mitigation mechanism can be utilized synergistically with other existing approaches in the form of three-dimensional soft scaffolds for Li plating, which achieves higher coulombic efficiency and better capacity retention than that for conventional copper substrates.”
Researchers included scientists from Rice University and Hunan University, China. Funding was provided in part by the Department of Energy.