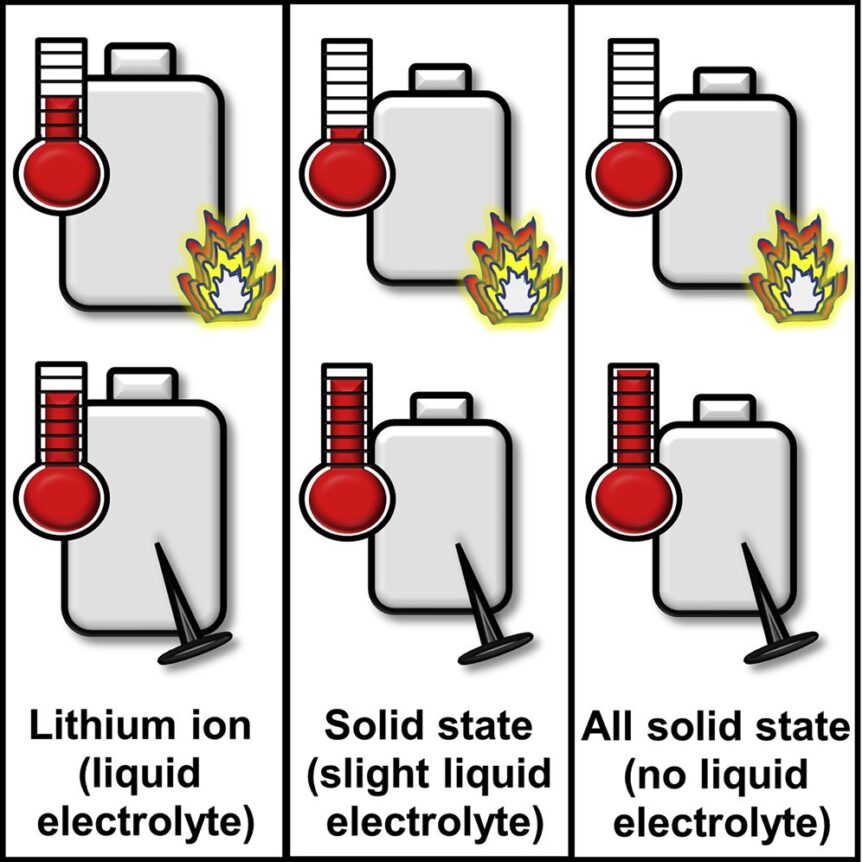
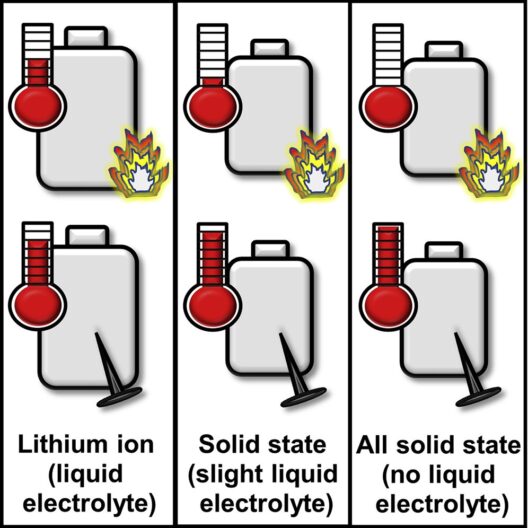
What if our assumptions prove wrong? How willing are we to at least examine closely-held beliefs and accept a new, scientific finding? Sandia engineers are demonstrating that solid-state batteries containing a little liquid electrolyte can be safer than conventional lithium-ion cells.

Sandia engineers Alex Bates, left, and John Hewson examine a lithium-ion battery in front of a specially designed battery testing chamber. They compared the heat released by a traditional lithium-ion battery to the heat released by a solid-state battery and a solid-state battery with a little liquid electrolyte. They found in many cases solid-state batteries with a little liquid electrolyte were safer than their lithium-ion counterparts. (Photo by Rebecca Gustaf)
PV Buzz included a great executive summary in their article on the findings:
- A new study tackled a long-held assumption that adding some liquid electrolyte to improve performance would make solid-state batteries unsafe.
- Instead, the research team found that in many cases solid-state batteries with a little liquid electrolyte were safer than their lithium-ion counterparts.
- They also found, if the battery were to short-circuit, releasing all its stored energy, the theoretically super-safe, all-solid-state battery could put out a dangerous amount of heat.
Those three points seem to contradict the conventional wisdom that eliminating electrolytes would lead to safer, less flammable batteries.
Sandia engineers Alex Bates and John Hewson have published findings challenging that conventional wisdom in the peer-reviewed journal Joule’s March 7 edition. Bates explained their findings. “Solid-state batteries have the potential to be safer, and they have the potential for higher energy density. This means, for electric vehicles, you could go farther in between charges, or need fewer batteries for grid-scale energy storage. The addition of liquid electrolyte may help bridge the gap to commercialization, without sacrificing safety.”
Abuse Testing Batteries
The researchers compare solid-state batteries to conventional lithium-ion batteries. “In both, lithium ions move from one side of the battery to the other, while electrons flow through a circuit to power the device. One big difference is that throughout a lithium-ion battery, there is a substance that helps the lithium ions move quickly: the liquid electrolyte.”
That liquid is often blamed for the catastrophic nature of lithium battery fires, which can be instigated from a number of causes.
Loraine Torres-Castro is a battery safety expert in Sandia’s Battery Abuse Testing Laboratory. She compares liquid electrolyte to a fleet of cars pulling into driveways: It shuttles lithium ions directly where they need to go. This valet parking of ions is subject to flammable outbreaks however, encouraging development of solid-state batteries in which eliminating the liquid electrolyte supposedly removes the fire danger.
Without that liquid, however, the car-parking analogy goes awry. Electrons become more like a line of trains, quickly getting to the station, but requiring the passenger ions to hop off and walk to their individual destinations.
The researchers took a cue from Yuliya Preger, a Sandia battery reliability expert on the project, Steve Harris, a battery scientist at Lawrence Berkeley National Laboratory, and Katie Harrison, a Sandia battery scientist. They questioned the “party line” that any amount of liquid electrolyte was dangerous.
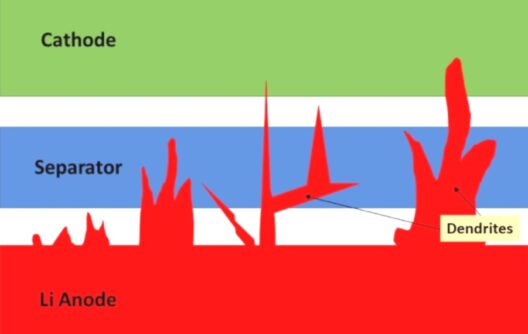
All lithium batteries can become heated and even explode. The lithium, separators and other factors play important parts in a battery’s stability, and some liquid electrolyte can reduce combustion in some cases.
The team reasoned that adding “a little bit” of liquid electrolyte to the positive side of the battery would speed up the “direct shuttling.” Batteries would have better charging speeds and performance. Just how much liquid was enough without creating a more volatile battery? They calculated how much heat would be released by each battery type in each failure mode. They compared heat release for a lithium-ion battery, an all-solid-state battery and solid-state batteries with varying amounts of liquid electrolyte.
John Hewson explained, “We started by determining just how much chemical energy is in the three kinds of batteries. “There’s only so much energy you can release, which will heat up the battery a certain amount, if a chemical reaction does occur.”
Lorraine Torres-Castro added that batteries could catch on fire from either a neighboring battery or a surrounding building. Researchers found that the solid-state battery with a little liquid electrolyte in it produced about one-fifth of the heat of a comparable lithium-ion battery, depending on how much liquid electrolyte it had. The solid-state battery without liquid electrolyte didn’t produce any heat under this scenario.
Yulia Preger notes that repeated charging and discharging might cause the lithium metal to form a “spike” called a dendrite, which can punch a hole through the separator between the two sides and cause a short-circuit. According to the researchers, this is a known issue with all batteries that have lithium metal on one side. In this case, all three batteries produced similar amounts of heat, which depended on how much lithium metal was in the batteries.
What if the solid electrolyte were to break? It the battery is crushed or punctured, or experiences built-up pressure during operation, the two sides of the battery might react. The solid state battery without electrolyte reached temperatures near that of lithium-ion battery under such conditions, “which the team found surprising.” Yulia Preger said that such breakages, “…Highlighted the importance of engineering the heck out of that separator so it does not fail.”
The team will conduct similar calculations with other electrolyte materials, conduct further experiments and validate the new and original calculations. Alex Bates concludes, “We found if the solid-state battery has lithium metal, it has the potential to be dangerous, regardless of if it has liquid electrolyte or not…. there’s a definite trade-off between performance and safety, but adding a bit of liquid may greatly increase performance while only having a small impact on safety.”
This may speed the way to commercialization. Loraine Torres-Casto sees hope in the research. “Having the clarity and the confidence that knowing a small amount of liquid electrolyte will not create huge safety issues may help the development of commercial solid-state batteries. The adding liquid electrolyte could fix one of their main problems, the solid electrolyte interface.”
This safety study was supported by DOE’s Office of Electricity Energy Storage Program.