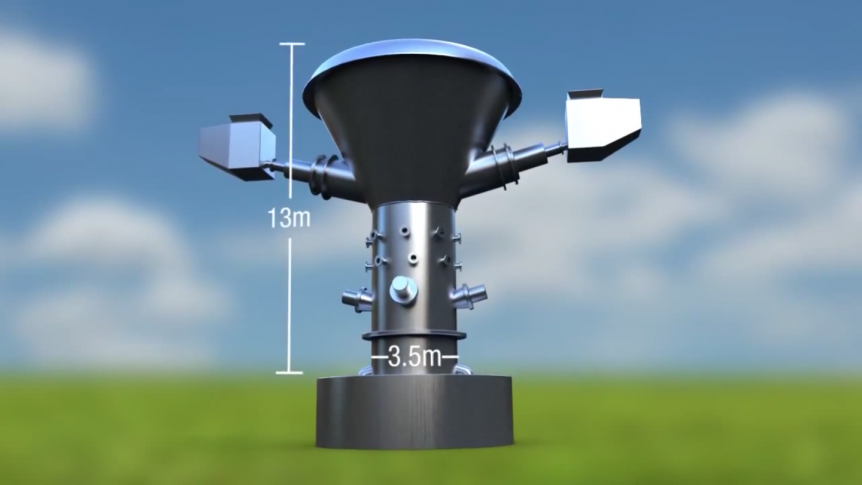
Dawn One is a solar-powered climate observatory, one of many to come and an outgrowth of a long career for John Langford, Electra Aero’s CEO, and collaborator with Professor James G. Anderson of Harvard University. A seeming callback to John Langford’s human-powered aircraft from his MIT days, Dawn One is a 90-foot span unmanned aircraft system (UAS) destined to fly at stratospheric altitudes (49.000 feet maximum) while observing data for quantitative forecasts of risks in the climate. We see its first flight from the Manassas Regional Airport in Virginia on September 9. The assistants in hot pink and orange vests are Hokies, part of Virginia Tech University, and whose name is explained in a lengthy Wikipedia entry. The “solar-battery hybrid electric research aircraft” is part of the Stratospheric Airborne Climate Observatory System (SACOS) program. The program will consist of “an ensemble of solar powered aircraft operating for months in the stratosphere,” each “ each “focused on critical climate observing missions …
Making Greener-Than-Green Hydrogen
What if we could make “clean” hydrogen from plain water, with none of the problems associated with coal, oil or gas extraction and the waste byproducts produced in extracting brown or blue H2? Several approaches such as artificial leaves have been developed, but a totally different new approach seems incredibly promising. A promising approach may lead to greener-than-green hydrogen. The Race to Invent the Artificial Leaf Varun Sivaram, in his book Taming the Sun discusses how Nate Lewis at Caltech and Daniel Nocera at Harvard “determined to find a way to wring fuel out of thin air.” Each has created an “artificial leaf” that emulates the photosynthesis performed naturally by real leaves. A real leaf is far more complex than one would imagine, and the chemical interactions inside even more so. For all that, photosynthesis is only one percent efficient. “A commercially viable artificial leaf would solve several of the trickiest challenges in clean energy. It would create a way …
Cambridge’s Artificial Leaf Makes Syngas
Cambridge University researchers have developed a new “artificial leaf” that uses sunlight, carbon dioxide and water to directly generate “syngas,” without releasing additional CO2 into the atmosphere. As the Cambridge team reported, ‘Syngas is currently made from a mixture of hydrogen and carbon monoxide, and is used to produce a range of commodities, such as fuels, pharmaceuticals, plastics and fertilizers.” Other “leaves” have been devised, perhaps the most famous being that of Daniel Nocera, formerly at MIT and now at Harvard University. He was among the first and at the time, most successful, or the leaf producers. Note the next step he proposes at the end of the short video. Professor Erwin Reisner from Cambridge’s Department of Chemistry has been working for seven years on achieving Nocera’s desire to directly produce fuel from the elements. “You may not have heard of syngas itself but every day, you consume products that were created using it. Being able to produce it sustainably …
Daniel Nocera Returns to the Artificial Leaf
Many scientists are turning to mimicking nature to probe its secrets, but Daniel Nocera, the Patterson Rockwood Professor of Energy at Harvard University, has gone far beyond his natural model. Reported in 2012, Nocera came up with the idea of an “artificial leaf,” a silicon sheet with a layer of cobalt-based catalyst that releases oxygen on one side and a layer a nickel-molybdenum-zinc alloy on the other side that releases hydrogen. Several researchers have followed this initial breakthrough, trying different materials and combinations of ingredients. For a while, it looked as though Nocera turned his attention to battery development, but recent news shows he’s back investigating artificial leaves – with great improvements over his initial efforts – and those of nature, it would seem. His newest approach combines the catalytic energy of the original leaf with a bacterium that makes useful fluids out of the hydrogen generated. It makes the leaf’s output a practical liquid – a fuel. It probably …
Cheap Hydrogen, Anyone?
Researchers in Glasgow and at Stanford University have devised ways to decouple oxygen and hydrogen from water without resort to expensive extraction or storage techniques. Both breakthroughs involve low-cost materials, low-energy requirements, and the production of clean hydrogen through what should be renewable energy resources. The latter overcomes one major objection to hydrogen production. As Professor Lee Cronin of the University of Glasgow’s School of Chemistry explains, “Around 95% of the world’s hydrogen supply is currently obtained from fossil fuels, a finite resource which we know harms the environment and speeds climate change. Some of this hydrogen is used to make ammonia fertilizer and as such, fossil hydrogen helps feed more than half of the world’s population. “The potential for reliable hydrogen production from renewable sources is huge. The sun, for example, provides more energy in a single hour of sunlight than the entire world’s population uses in a year. If we can tap and store even a fraction of …
New “Leaf” Turns Over More Energy
Scientists have been working on imitating nature’s ability to photosynthesize the sun’s energy, much as plants turn that energy into food for their health and growth. Daniel Nocera, for instance, created an artificial leaf that split water into oxygen and hydrogen that could fire up a small fuel cell and run an electric light. According to a Science Pub lecture your editor recently attended, an eight-ounce glass of water can power a 60-Watt bulb for 20 hours. Nocera, in a Pop! Tech talk, claims an Olympic-size swimming pool could supply all the world’s energy needs. Nocera now works at Harvard, but researchers at Massachusetts Institute of Technology (MIT), his former home, are taking his work further, detailing all the limitations that keep his “artificial leaf” from giving off more storable energy. As explained in the MIT press release, “The original demonstration leaf, in 2011, had low efficiencies, converting less than 4.7 percent of sunlight into fuel… But the team’s new …