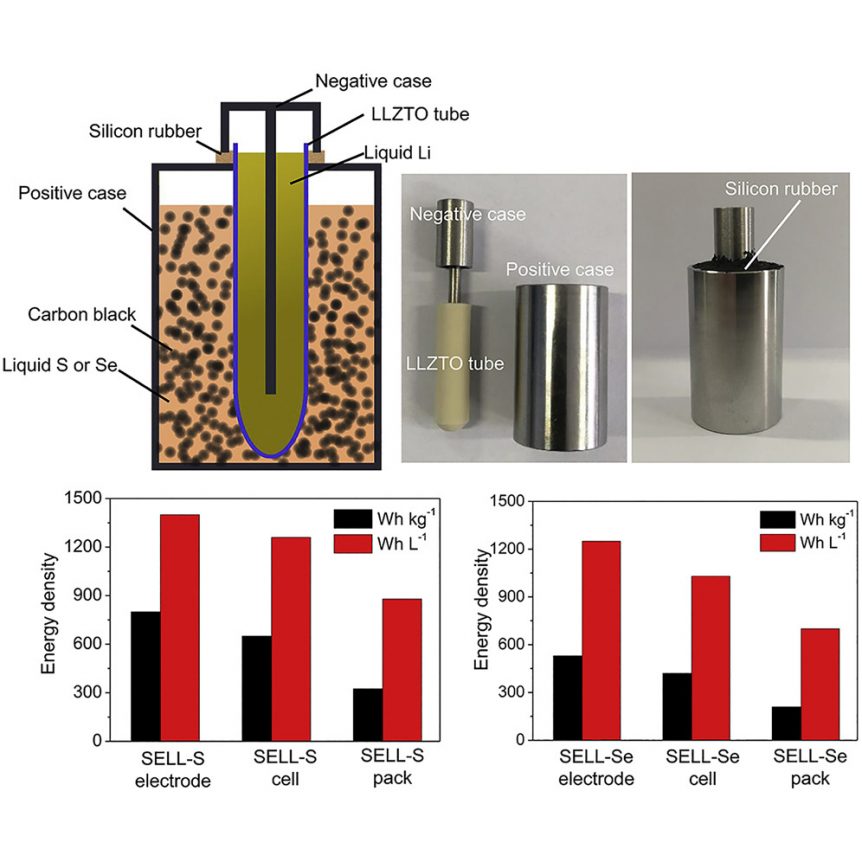
In battery making, recipes for electrolytes play an important part of the whole. In a new formula whipped up by Zhengzhou University, Tsinghua University and Stanford University, Lithium (Li), Lanthanum (La), Zirconium (Zr), Tantalum (Ta), and Oxygen (O) form a ceramic tube as the battery’s electrolyte. This tube is centered in new solid state Lithium Sulfur and Lithium Selenium batteries. Researchers filled that tube with a liquid lithium electrode, and immersed the tube in a bath of carbon black and liquid selenium or sulfur in a stainless steel container. The team’s paper, “High Energy-Density Solid Electrolyte-Based Liquid Li-S and Li-Se Batteries,” published in the October 15 edition of Joule, explains the new batteries should be capable of delivering energy densities of around 2,600 Watt-hours per kilogram for the lithium-sulfur chemistry and 1,160 Wh/kg. for lithium-selenium. Currently, researchers have achieve 500 Wh/kg, double the best Li packs today. This can be achieved at cost of $41 per kilowatt-hour for the Li-Se …
Tag Archive
Below you'll find a list of all posts that have been tagged as “liquid lithium electrode”