Hydrogen would be a nearly perfect fuel if it didn’t take more energy to extract it than you can get out of it. Scientists have been working for years to isolate it in an economical fashion. The most common element in the universe, hydrogen makes up 10 percent of the weight of living things here on earth – mainly in water, proteins and fats. Its bonds in water make it pervasive, but still distant. Obtaining it can be as simple as the video below. But the short bursts derived from this approach will exhaust the battery and not provide as much energy in return. Waste Not, Want Not Ironically, much of the earth’s other resources, more easily gained, are wasted in our society’s rush to consume. Recent reports show that up to a third of the food produced today goes to waste. Huge quantities of biomass could seemingly be put to good use rather than adding to the methane that …
Caging Silicon Anodes with Graphene
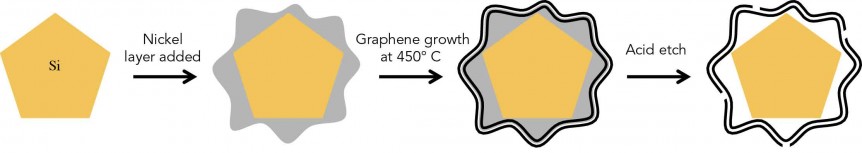
Dr. Yi Cui of Stanford University has expanded the idea of “battery” to include conductive ink on paper, fruit-like clusters of energy-storing capsules, and now, nano-sized graphene cages in which the energy can romp like a hamster in a plastic ball. He will be on hand at this year’s Sustainable Aviation Symposium on May 6, at the Sofitel San Francisco Bay hotel. His pioneering work with silicon as an electrode material goes back at least ten years, and has focused on overcoming silicon’s two major problems in battery use. Silicon expands and begins breaking down during repeated charge-discharge cycles. It reacts with battery electrolyte to form a coating that progressively destroys performance. The combination of crumbling and coating finally makes the battery inoperable. His group at Stanford had found a way to “wrap every silicon anode particle in a custom-fit cage made of graphene, a pure form of carbon that is the thinnest and strongest material known and a great conductor …