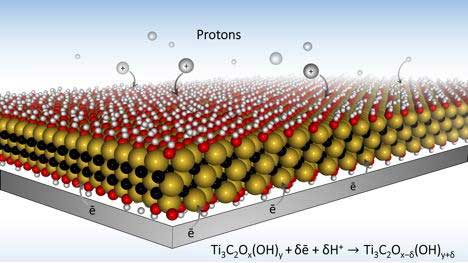
Over the years reporting on battery developments, we’ve seen paper batteries, spray-on batteries, structural batteries and many types of material mixes. Drexel University has tossed all the above intone big hopper and come up with MXene, a potentially dynamic way of making batteries, supercapacitors, antennas, and structural elements that can be conductors, semiconductors, and insulators, among myriad applications. Going Through a Phase MXenes are formed from layered MAX phases, defined by Drexel as forming, “A large family of ternary(composed of three) carbides with the general formula Mn+1AXn, where n = 1–3, M is an early transition metal, A is an A-group element (mostly IIIA and IVA), and X is C and/or N:” That level of chemistry is two quantum leaps above your editor’s pay grade, so you’ll have to work out the implications for yourself. Or, you can read the more understandable explanation in this link. Drexel explains, “MXenes are made by chemically etching a layered ceramic material called a MAX phase, to remove a set of chemically-related …
Tag Archive
Below you'll find a list of all posts that have been tagged as “Trinity College Ireland”