The best batteries as now produced use expensive materials and processes to achieve high energy density. Could a century-old idea be resurrected to provide an inexpensive alternative to today’s costly electric storage devices? Science Daily reports on a recent attempt to improve on a proven technology.
Stanford University’s Hongjie Dai, professor of chemistry and head of a research group, is working with the Edison battery, named for Thomas Alva Edison, and using the nickel-iron electrodes Edison favored, but with a modern twist to overcome one of its disadvantages.
Stanford’s news bulletin quotes Dai. “The Edison battery is very durable, but it has a number of drawbacks. A typical battery can take hours to charge, and the rate of discharge is also very slow.”
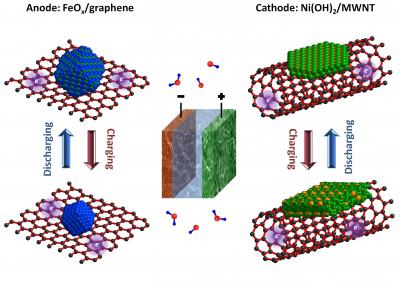
Powering electric vehicles in the early 1900s, Edison’s battery is used today in limited instances to store surplus electricity from solar panels and wind turbines where charging and discharge speeds are not a major consideration. Dai’s group started with graphene sheets and 10 nested carbon nanotubes – and “grew” nanocrystals of iron oxide onto the graphene and nanocrystals of nickel hydroxide onto the carbon nanotubes.
Edison’s original nickel cathode and iron anode, meant to replace more expensive lead-acid batteries, is now graced with modern nanotechnology and reacts much faster than the original. Science Daily reports, “The Stanford team has created an ultrafast nickel-iron battery that can be fully charged in about 2 minutes and discharged in less than 30 seconds. The results are published in the June 26 issue of the journal Nature Communications.
“’We have increased the charging and discharging rate by nearly 1,000 times,’ said Stanford graduate student Hailiang Wang, lead author of the study. ‘We’ve made it really fast.’”
“’Coupling the nickel and iron particles to the carbon substrate allows electrical charges to move quickly between the electrodes and the outside circuit,’ Dai said. ‘The result is an ultrafast version of the nickel-iron battery that’s capable of charging and discharging in seconds.’”
For now, the prototype one-Volt battery can light a small flashlight, but researchers hope to scale that to Nissan Leaf and Chevrolet Volt, or even to power grid size. Several issues might restrain it from becoming the budget salvation of EVs, however.
Wang added, “’Our battery probably won’t be able to power an electric car by itself, because the energy density is not ideal, but it could assist lithium-ion batteries by giving them a real power boost for faster acceleration and regenerative braking.’” The battery also lacks cycle life, dropping 20 percent in capacity after 800 charge/discharge “round trips”. Combining the battery’s low-cost ingredients with existing lithium chemistries may forge a hybrid cell that could exploit the advantages of both.
Wang noted big plus in safety. “It’s definitely scalable Nickel, iron and carbon are relatively inexpensive. And the electrolyte is just water with potassium hydroxide, which is also very cheap and safe. It won’t blow up in a car.”
Dai’s group was featured in this blog for their work on “unzipped” carbon nanotubes, another approach to reducing the cost of providing high energy density by replacing platinum catalysts in lithium batteries with lower cost carbon.