Batteries are complex things to design and make, with materials scientists and chemists facing unlimited numbers of options for materials choices, formulations and proportions, and manufacturing techniques that will make hoped-for performance attainable on a commercial level.
Yi Cui and a distinguished array of undergraduate and graduate students at Stanford University have written 320 academic research papers since 2000, with the rate of publication seeming to increase every year. To put icing on that multi-layered cake, Dr. Cui has helped found his own battery company, Amprius, using his depth of knowledge to take batteries in directions interesting enough to draw the attention of well-known investors – including Stanford. The only recent information on the web site today shows the firm is looking for a battery scientist and a battery engineer.
Carbon matrix infused with lithium spreads metal into large, porous surface with considerably greater energy capacity
His academic and research work continue, though, with his latest efforts producing a turn away from his work with silicon – ,making a novel lithium/carbon electrode with extremely high volumetric and gravimetric capacities. Green Car Congress says “Lithium-metal anodes are favored for use in next-generation rechargeable Li-air or Li-sulfur batteries due to a tenfold higher theoretical specific capacity than graphite (3,860 mAh/g vs. 372 mAh/g); light weight and lowest anode potential. However, safety issues resulting from dendrite formation and instability caused by volume expansion have hampered development and deployment of commercially viable solutions.”
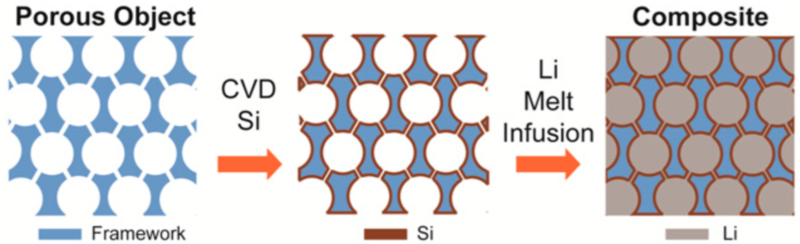
Dr. Cui’s team has apparently addressed the issues by “encapsulating lithium inside a porous host scaffold using a facile melt-infusion approach. Uniformly confined within the matrix, the lithium creates a material that can deliver a high capacity of around 2,000 mAh/g (gravimetric) or 1,900 mAh/cm3 (volumetric) as stable anodes for Li-metal batteries.”
A team at Stanford led by Professor Cui has now introduced a simple approach to address both issues by effectively encapsulating lithium inside a porous host scaffold using a facile melt-infusion approach. Uniformly confined within the matrix, the lithium creates a material that can deliver a high capacity of around 2,000 mAh/g (gravimetric) or 1,900 mAh/cm3 (volumetric) as stable anodes for Li-metal batteries.
Researchers were investigating several facets of achieving high outputs with the metal/composite electrode:
- mechanical and chemical stability toward electrochemical cycling;
- low gravimetric density to achieve high-energy density of the composite anode;
- good electrical and ionic conductivity to provide unblocked electron/ion pathway, enabling fast electron/ion transport; and
- relatively large surface area for Li deposition, lowering the effective electrode current density and the possibility of dendrite formation.
Using porous carbon-based materials – in this case an “electrospun carbon fiber network – the team coated it with a lithiophilic (able to attract lithium) material, and then infused molten lithium (which melts at 180° C, or 356° F) into the composite host.
This gave them the results they were looking for. In a previous experiment using zinc to attract lithium on to the electrode as an active material, they explained:
” Lithium metal is the ideal anode for the next generation of high-energy-density batteries. Nevertheless, dendrite growth, side reactions and infinite relative volume change have prevented it from practical applications. Here, we demonstrate a promising metallic lithium anode design by infusing molten lithium into a polymeric matrix.”
Again, the team is creating a composite electrode that seems to hold together even while storing high levels of energy. Compared to a “hostless” lithium metal anode, the litium/carbon composite handles swelling during charging and shrinkage during discharge better, mitigating the “potential safety hazard.” Having a larger surface area reduces current density and “triggers a greatly improved electrochemical performance.”
Initial testing of only 80 charge-discharge cycles (1,000 to 2,000 cycles would be more like the lifetime an anode faces in the real world), shows stable cycling of over 2,000 milliamp-hours per gram at a high current density of three milliamps per square centimeter.
That theoretical gravimetric specific capacity of a lithium metal of 3,860 mAh/g is “diluted” by the fact that the lithium is embedded in a matrix of other material and amounts to only 60 percent of the total anode. By the team’s calculations, 60 percent of 3,860 is 2,316 mAh/g (gravimetric), close to the measured value for the test anode of 2,061 mAh/cm3 (volumetric)
A paper on their work is published in Proceedings of the National Academy of Sciences (PNAS). Zheng Liang, Dingchang Lin, Jie Zhao, Zhenda Lu, Yayuan Liu, Chong Liu, Yingying Lu, Haotian Wang, Kai Yan, Xinyong Tao, and Yi Cui contributed to the paper. You can see the entire paper here.