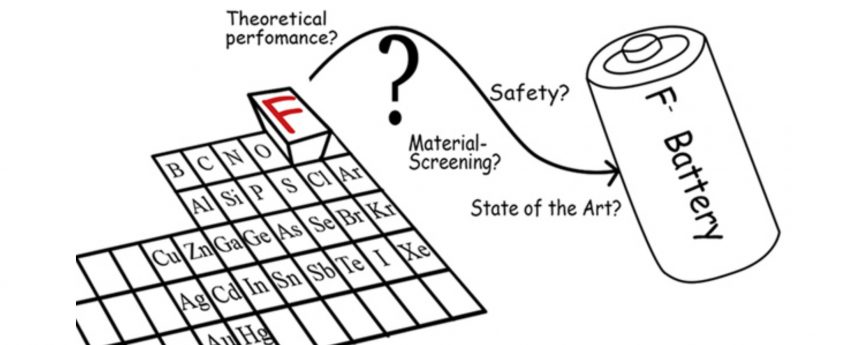
Can the stuff that protects your teeth find happiness storing electrical energy? Researchers are brushing up on their chemistry to produce a fluoride battery – almost the total opposite of a lithium battery. Three years ago, The Journal of Fluorine Chemistry quibbled, “Only a handful of publications exist on the topic of fluoride ion batteries (FIBs). These are electrochemical cells in which a negative anion—fluoride—enables charge transport. In this review, we will report, for the first time, an extensive theoretical screening of FIBs as well as an analysis of the safety and toxicity of electrochemical couples of such batteries.” It continued with an exploration of high-temperature (150° C, or 302° F) and room-temperature examples of fluoride cells and ended with comparisons of seven different cathode and nine different anode materials “to further illustrate the potential and issues of such battery systems.” Now, Honda, researchers from the California Institute of Technology (CalTech), NASA’s Jet Propulsion Laboratory and a scientist now at …
Tag Archive
Below you'll find a list of all posts that have been tagged as “Dr. Christopher Brooks”