Two groups of researchers in South Korea and America have added silicon and sugar as ways of gaining more energy from two different types of batteries.
The 10X Battery at Last?
Researchers at Pohang University of Science & Technology (POSTECH) in South Korea have developed a “layering-charged, polymer-based stable high-capacity anode material.” POSTECH professors Soojin Park (Department of Chemistry) and Youn Soo Kim (Department of Materials Science and Engineering) and Professor Jaegeon Ryu (Department of Chemical and Biomolecular Engineering) of Sogang University think their discovery could increase current electric vehicle range “at least 10-fold.”
As noted in this blog many times, the idea of a 10X battery has been a matter of intense research from Yi Cui at Stanford and his research partner Jaephil Cho in South Korea, along with John Goodenough at Rice University and Jeff Dahn at Canada’s Dalhousie University – among others.
Silicon and Polymeric Benders
One of the bigger problems with using silicon in a battery is its need to expand as it is charged, then contract as it is discharged. Constant cycling in a rechargeable battery leads to deterioration of the silicon and eventual failure. Decades of efforts have come to at least partial solutions. Dr. Cui’s Amprius Batteries, for instance, has devised silicon nanowires to counter the destruction and failure.
POSTECH researchers are betting their layered look will help overcome silicon’s problems. Their “charged polymeric binder” combines a weak link (hydrogen bonding) with a strong link (chemical crosslinking). These are apparently rarely combined, but in POSTECH’s approach, brought together.
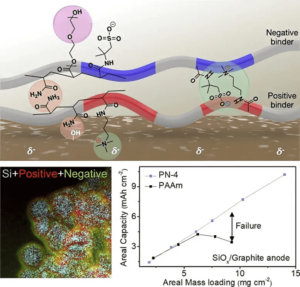
POSTECH’s layering approach enables higher output, lower failure rate
The resulting “stable, high-capacity anode material” is held together with a “functional polymeric binder” that could “increase current EV range at least 10-fold.” The anode material replaces graphite with silicon and layers of charged polymers.
The research results were published as the Front Cover Article in Advanced Functional Materials.
The abstract for the paper includes these more astute points: “Chemical crosslinking involves covalent bonding between binder molecules, making them solid but has a fatal flaw: once broken, the bonds cannot be restored. On the other hand, hydrogen bonding is a reversible secondary bonding between molecules based on electronegativity differences, but its strength (10-65 kJ/mol) is relatively weak.
“The new polymer developed by the research team not only utilizes hydrogen bonding but also takes advantage of Coulombic forces (attraction between positive and negative charges). These forces have a strength of 250 kJ/mol, much higher than that for hydrogen bonding, yet they are reversible, making it easy to control volumetric expansion. The surface of high-capacity anode materials is mostly negatively charged, and the layering-charged polymers are arrayed alternately with positive and negative charges to effectively bind with the anode. Furthermore, the team introduced polyethylene glycol to regulate the physical properties and facilitate Li-ion diffusion, resulting in the thick high-capacity electrode and maximum energy density found in Li-ion batteries.”
A Little Bit of Sugar Makes the Battery Stay Up
Adding sugar to the anolyte, mix in a flow battery has had unexpected results for Pacific Northwest National Laboratory researchers.
Flow batteries have been around since at least 1868, and were the second type of energy-storage system to power an electric aircraft, the dirigible LaFrance floating over the French countryside in 1884. Although the LaFrance battery weighed almost half a ton, it managed to propel the huge vehicle around the skies for several accumulated hours.
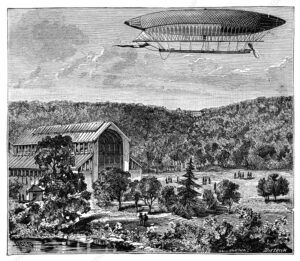
‘La France’ electric airship. 19th-century illustration of the launch on 9 August 1884 of the electric airship ‘La France’ from its hanger at the Chalais-Meudon aerodrome near Paris, France. This 52-metre-long hydrogen-filled airship was designed by French engineers Charles Renard and Arthur Krebs. Piloted by Krebs, the airship covered 8 kilometers in 23 minutes. This was the first ever fully controlled free-flight, returning under control to its starting point.
Regrettably flow batteries are typically large for their output, taking up more room than other chemically-based energy storage systems. This makes them great for ground-based operations, such as maintaining electric service at night when the wind dies down or the sun disappears until tomorrow morning.
PNNL has been able to accomplish a great deal with reducing the size of flow batteries, producing a “record-lifetime aqueous organic redox (reduction/oxidation) flow battery.” Researchers promoted “Proton-regulated alcohol oxidation for flow battery kinetic acceleration” by adding a chemical sugar (β-cyclodextrin) to the mix. This speeds up ion shuttling in the battery.
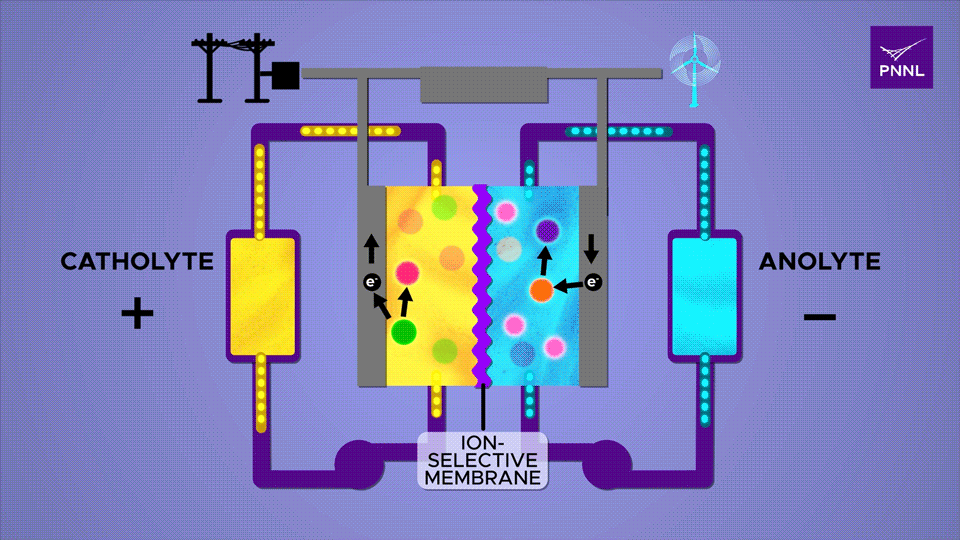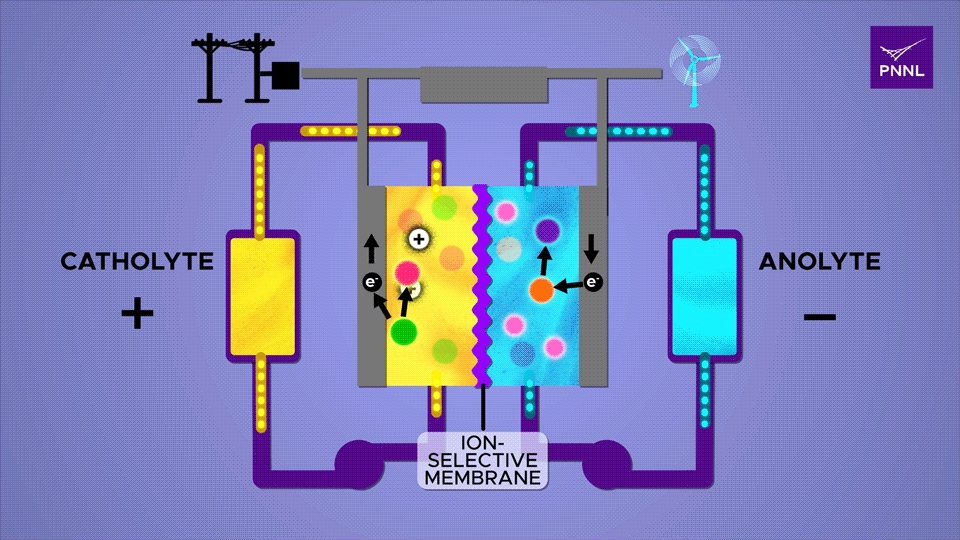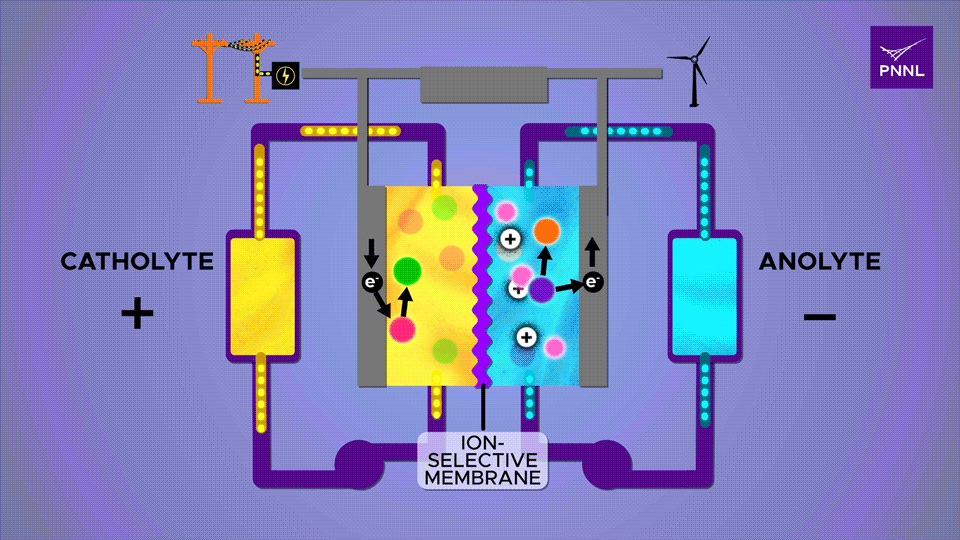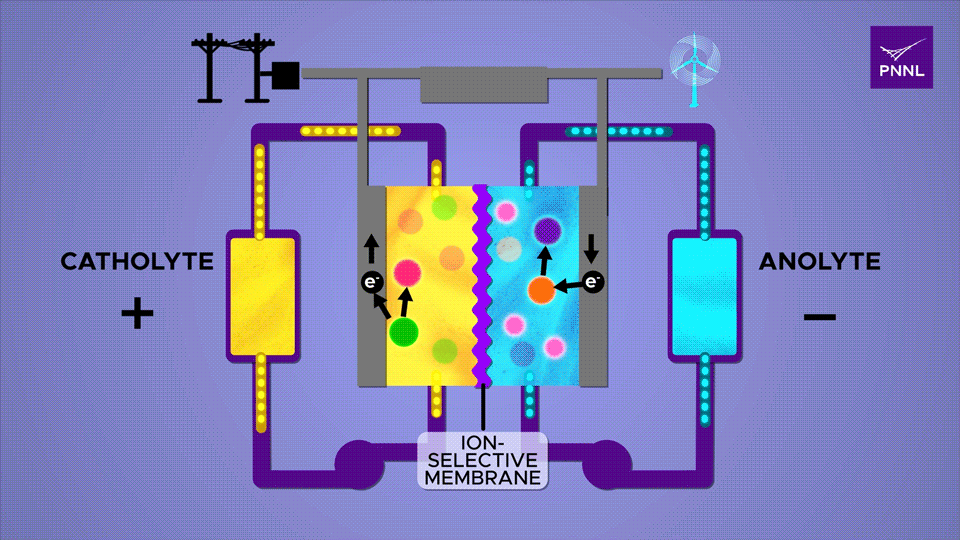
An ion-selective membrane allows positively charged ions to move between the two electrolytes during charge and discharge cycles, causing electrons to run through the outer plates and through a circuit. The β-cyclodextrin additive, a derivative of starch, here shown in pink, greatly accelerates this reaction Sara Levine / PNNL
The abstract from the Joule entry explains this all in more scientific terms. “Redox flow batteries have a unique architecture that potentially enables cost-effective long-duration energy storage to address the intermittency introduced by increased renewable integration for the decarbonization of the electric power sector. Targeted molecular engineering has demonstrated electrochemical reversibility in natively redox-inactive ketone molecules in aqueous electrolytes. However, the kinetics of fluorenone-based flow batteries continue to be limited by slow alcohol oxidation. We show how strategically designed proton regulators can accelerate alcohol oxidation and thus enhance battery kinetics. Fluorenone-based flow batteries with the organic additive β-cyclodextrin demonstrate enhanced rate capability, high capacity, and long cycling. This study opens a new avenue to improve the kinetics of aqueous organic flow batteries by modulating the reaction pathway with a homogeneous catalyst.”
