Graphene is fascinating stuff, with tons of promise. Whether it can produce usable results for energy storage is still an open question. But Samsung and a startup called Real Graphene may have at least a start in that realm. GAC, a Chinese auto firm, seems to be promising batteries before the end of what is left of this year. There may be hope in the midst of a dark winter. Samsung’s Lagging Promise In 2017 a team of researchers at the Samsung Advanced Institute of Technology (SAIT) developed a “graphene ball,” promising a 45-percent increase in capacity, and five times faster charging speeds than standard lithium-ion batteries. Hoping to power mobile devices and electric vehicles, SAIT collaborated closely with Samsung SDI* as well as a team from Seoul National University’s School of Chemical and Biological Engineering. *Not a simple abbreviation, SDI stands for “Samsung with the initial letter S, ‘Display’ and ‘Digital’ with D and ‘Interface’ and ‘Internet Component’ with …
Tripling Down on Cathode Capacity
intercalation (countable and uncountable, plural intercalations) (chemistry) The reversible insertion of a molecule between two others. Wiktionary Enyuan Hu, a chemist at Brookhaven National Laboratory explains the importance, and limitations, of intercalation in battery chemistry. “The materials normally used in lithium-ion batteries are based on intercalation chemistry. This type of chemical reaction is very efficient; however, it only transfers a single electron, so the cathode capacity is limited. Some compounds like FeF3 are capable of transferring multiple electrons through a more complex reaction mechanism, called a conversion reaction.” Iron trifluoride (FeF3) is composed of “cost-effective and environmentally benign elements — iron and fluorine. Researchers have been interested in using chemical compounds like FeF3 in lithium-ion batteries because they offer inherently higher capacities than traditional cathode materials,” according to Brookhaven. Scientists at the University of Maryland (which led the research), Brookhaven and the U.S. Army Research Lab developed and studied the FeF3 cathode. Xiulin Fan, a scientist at UMD and one of the lead authors of …
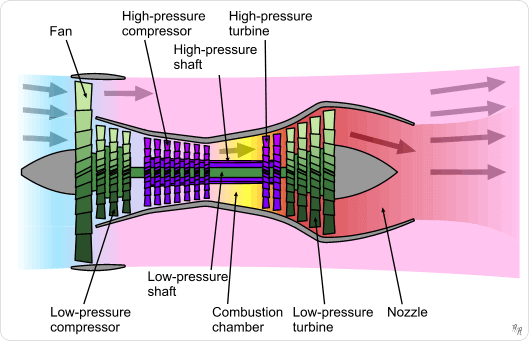
Hotter Jet Engines Could Lead to Greener Flight
Nano twins are not something Robin Williams’ Mork would make friends with. They are destructive pairings inside alloys, and getting rid of them will apparently lead to cleaner jet flight. Ohio State University researchers devised a technique they call “Phase Transformation Strengthening” which leads to stronger alloys and less deformation of the final products. This is good news for jet engine and turbine designers, since an engine that can run hotter will burn its fuel more completely, resulting in a less toxic exhaust. Nano twins “are microscopic defects that grow inside alloys and weaken them,” according to Ohio State University researchers. These defects weaken and deform an alloy when it is exposed to heat and pressure – two things present in a jet engine or power turbine. Michael Mills, professor of materials science and engineering and leader of the project at Ohio State, led the research. “We found that increasing the concentrations of certain elements in super-alloys inhibits the formation …
Samsung Almost Doubles Li-Ion Battery Capacity – in the Near Future
Several sources report on Samsung’s announcement that they have developed a new technology that enables them to coat silicon battery cathodes with high crystal graphene, virtually doubling the capacity of lithium-ion batteries. Of course, Samsung relates this immediately to their popular smartphones and tablets, but the significance of this is not lost on electric vehicle designers. Doubling the range of EVs “without adding a single pound of weight” would be a true game changer. But don’t get excited too quickly. Silicon electrodes have been a major research effort for people like Dr. Yi Cui, who spoke at this year’s Electric Aircraft Symposium. Issue surrounding their successful use have included silicon’s expansion when being charged and contraction when being discharged. This errant flexibility causes eventual disintegration of the electrodes and shuts down the battery. Attempts to use silicon nanowires still have led to embrittlement. Kompulsa.com reports Cho Jin-young from BusinessKorea explaining, “Currently, the development of high-capacity battery materials has been mostly done …
High-capacity, Soft Batteries From Trees
This is not pulp fiction, but pulp fact, trees being converted into squishy new nerf-like batteries. Researchers at Sweden’s KTH Royal Institute of Technology and Stanford University have made elastic, high-capacity batteries from wood pulp. The foam-like battery material can withstand shock and stress, according to the schools. Max Hamedi, a researcher at KTH and Harvard University, says, “It is possible to make incredible materials from trees and cellulose.” The wood-based aerogel material can be used for three-dimensional structures, important for overcoming certain restrictions imposed by two-dimensional approaches. Hamedi explains, “There are limits to how thin a battery can be, but that becomes less relevant in 3D. We are no longer restricted to two dimensions. We can build in three dimensions, enabling us to fit more electronics in a smaller space.” Moving past former obstacle of using three-dimensional, porous materials in crafting electrodes, researchers managed to make this a non-problem. Hamedi adds, “In fact, this type of structure and material …
Bulletproof Batteries?
Researchers announce that a “New battery technology from the University of Michigan should be able to prevent the kind of fires that grounded Boeing 787 Dreamliners in 2013.” The use of the word “should” is instructive, since scientist usually couch such announcements in more guarded terms. Battery separator materials are usually not the glamorous part of cell development, most headlines given to electrode and electrolyte breakthroughs. Kevlar may be a way, within batteries, of preventing a breakthrough. Nanofibers extracted from Kevlar, that impenetrable material in bullet-proof vests, “stifles the growth of metal tendrils that can become unwanted pathways for electrical current,” according to a University of Michigan report. Separator material stands between layers of other battery materials and ideally allows the passage of ions between electrodes in the battery. As the researchers report, “The innovation is an advanced barrier between the electrodes in a lithium-ion battery.” The nanofiber material has openings large enough to allow transfer of ions between anode …
Ohio State’s Solar-Air Potassium Battery
Ohio State University researchers have come up with a two-in-one solar cell/battery combination that promises great efficiency and low costs. What’s not to like? Unfortunately for readers of the blog, it’s initially only a stationary system that will make energy storage a viable circumstance for large power plants, but it seems that the technology could be adapted to lighter, portable applications, such as electric vehicles. Ohio State is keeping somewhat mum about the patent-pending device, which they are developing as a commercial entity under the auspices of their spin-off, Kair (K for potassium, plus air and pronounced “care”). We’ve heard a great deal about upcoming lithium-air batteries, but potassium-air is unique. Even more unique, this battery stores energy from its own solar cell, the world’s first solar battery. A mesh solar panel allows air to enter the battery, and a special process transfers “electrons between the solar panel and the battery electrode. Inside the device, light and oxygen enable different …
Raising Cane at the Battery Works
What if a battery could be made with higher energy and power densities than those currently available, while exploiting a natural material that’s both abundant, recyclable and inexpensive? Last year, the blog reported on Y. H. Percival Zhang’s work with xylose, a sugar found in most plants, to make hydrogen that could be used in fuel cells. Dr. Zhang, with a Ph.D. in chemical engineering and biotechnology from Dartmouth University, draws on his unique pair of specialties to inspire his forays into developing novel ways of extracting energy from natural sources. His latest effort is a battery that runs on maltodextrin, a polysaccharide made from the partial hydrolysis of starch. That starch can be derived from almost any type of plant, a ubiquitous and non-food-based source. This makes for a tidy life cycle, extracting the raw materials from nature and being able at the end of the battery’s long and refillable run to return them to nature without fear of …
Hybrid Batteries in Hybrid Vehicles?
Frances White of the Pacific Northwest National Laboratory (PNNL) reports that a new anode quadruples the life of a test lithium-sulfur battery and could lead to much lower costs for electric vehicles and large-scale energy storage. This blog has noted that many researchers focus on development of better cathodes, or anodes, or electrolytes exclusively, neglecting a more holistic, or whole battery approach to their delving. PNNL scientists have a reason for focusing on anodes, having found that a “battery with a dissolved cathode can still work.” What dissolves the electrodes in a battery? “Unwanted side reactions,” according to PNNL, cause the battery’s sulfur-containing cathode to disintegrate slowly and form polysulfide molecules that dissolve into the battery’s electrolyte liquid. This becomes a thin film that forms on the surface of the lithium-containing anode, and grows until the battery will no longer operate. Rather than trying to stop sulfur leakage from the cathode as others have, PNNL added a protective graphite shield …
Cheap and Dirty Fuel Cells – Good News for Hydrogen
Hydrogen fuel cells would be just about the most wonderful power producers in the world if they weren’t so expensive and so finicky about their diet of hydrogen. Their catalysts, usually made of costly platinum, can be destroyed by impurities in the gas. Making high-purity hydrogen is an exacting task and adds to the expense of operation. Two studies by scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory; one in conjunction with researchers at South Korea’s Ulsan National Institute of Science and Technology (UNIST), Korea Institute of Energy Research (KIER), show that it may be commercially possible to make less expensive catalysts with available materials, and in one case, use “dirty” hydrogen that would otherwise destroy fuel cells. The reduced price of making such hydrogen would further add to operational economies. Green Car Congress reports that Brookhaven and UNIST have discovered, “a new family of non-precious metal catalysts based on ordered mesoporous porphyrinic carbons (M-OMPC) with high …
- Page 1 of 2
- 1
- 2